
USA Pharmaceutical Market Outlook to 2030
Region:North America
Author(s):Naman Rohilla
Product Code:KROD2818
November 2024
84
About the Report
USA Pharmaceutical Market Overview
- The USA pharmaceutical market is valued at around USD 598 billion, driven by several key factors including the increasing demand for innovative drugs and the expanding prevalence of chronic diseases such as diabetes, cancer, and cardiovascular disorders. Pharmaceutical giants continue to invest heavily in research and development (R&D), ensuring a consistent pipeline of new and advanced medications. The U.S. market also benefits from a strong regulatory framework, such as the FDAs accelerated approval processes, which support the rapid introduction of life-saving therapies to the market.
- Cities like Boston, San Francisco, and New York dominate the pharmaceutical market due to their robust biopharmaceutical ecosystems, world-class research institutions, and access to capital. Boston, in particular, serves as a hub for biotech startups and large pharmaceutical companies, given its proximity to universities like Harvard and MIT. San Franciscos prominence is attributed to Silicon Valleys influence, fostering collaborations between biotech firms and tech companies working on healthcare innovations.
- The Inflation Reduction Act, implemented in 2023, aims to increase drug price transparency in the USA. The legislation requires pharmaceutical companies to publicly disclose the rationale behind price increases and gives Medicare the authority to negotiate drug prices directly. These initiatives are expected to reduce drug costs for consumers and make essential medicines more accessible, improving overall healthcare affordability.

USA Pharmaceutical Market Segmentation
The USA Pharmaceutical Market is segmented by product type, therapeutic area, distribution channel, application, and region.
By Product Type: The USA pharmaceutical market is segmented by product type into small molecule drugs, biologics, generics, and biosimilars. Biologics hold the dominant share in the market due to their ability to treat complex conditions such as cancer and autoimmune diseases, which require targeted therapies. With advancements in biotechnology, biologics have seen strong demand, particularly for personalized medicine approaches. Companies like Pfizer and Amgen have been pivotal in expanding the biologics segment, capitalizing on their R&D efforts and regulatory approvals from the FDA.

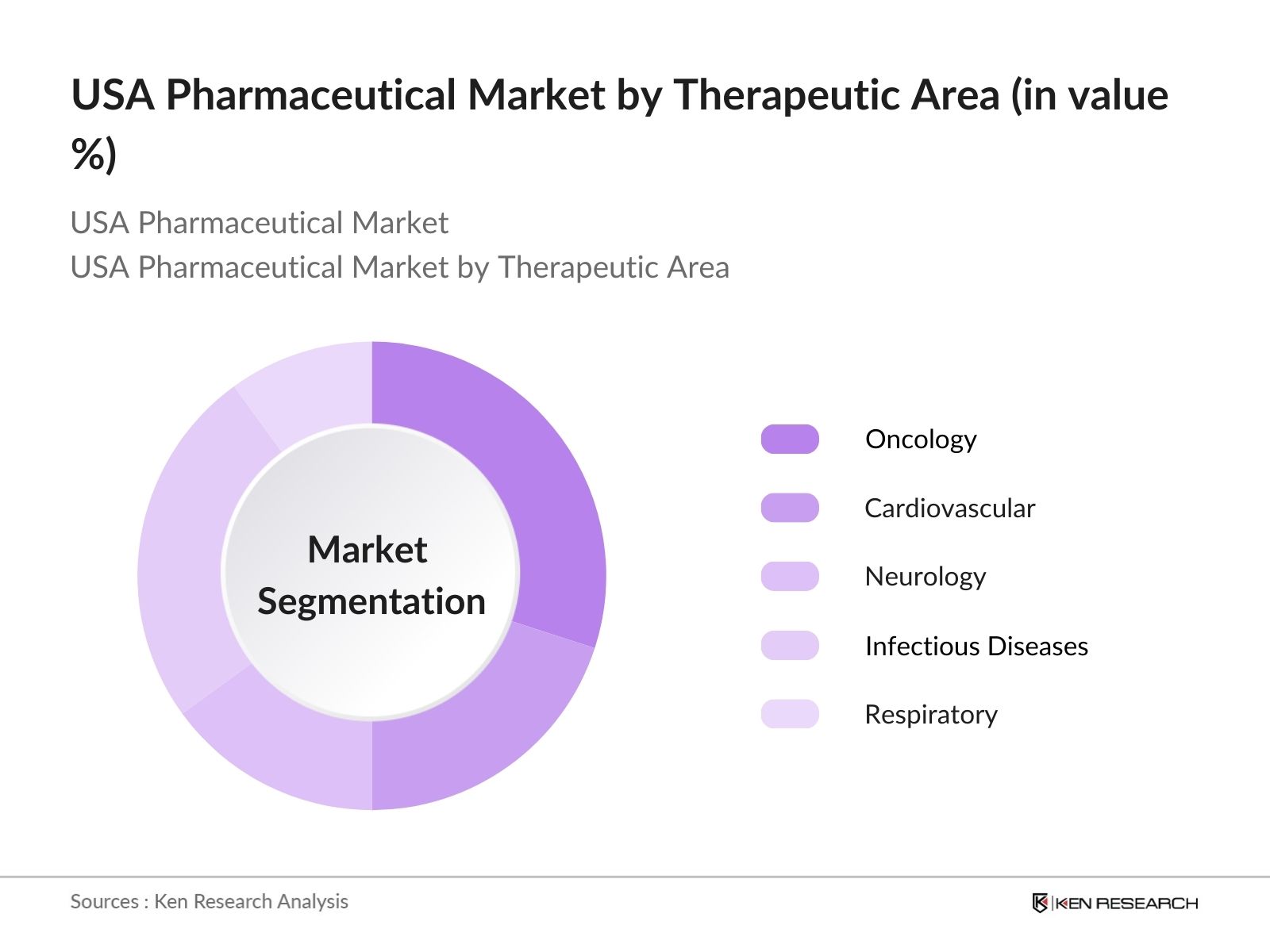
By Therapeutic Area: The USA pharmaceutical market is also segmented by therapeutic areas, including oncology, cardiovascular, neurology, infectious diseases, and respiratory. Oncology leads the therapeutic areas, driven by the increasing prevalence of cancer and the continuous introduction of innovative therapies.
USA Pharmaceutical Market Competitive Landscape
The USA pharmaceutical market is dominated by a few key players that maintain leadership through aggressive R&D spending, regulatory approvals, and strategic partnerships. Major pharmaceutical companies such as Pfizer, Johnson & Johnson, and Merck & Co., Inc. are instrumental in developing both innovative small molecules and biologics. Competition within this market is intense, and consolidation through mergers and acquisitions is common, as companies seek to expand their product portfolios and market reach.
|
Company |
Establishment Year |
Headquarters |
Revenue (USD) |
R&D Expenditure (USD) |
Key Focus Area |
Patent Portfolio |
Pipeline Products |
FDA Approvals |
|
Pfizer Inc. |
1849 |
New York, USA |
- |
- |
- |
- |
- |
- |
|
Johnson & Johnson |
1886 |
New Brunswick, USA |
- |
- |
- |
- |
- |
- |
|
Merck & Co., Inc. |
1891 |
Kenilworth, USA |
- |
- |
- |
- |
- |
- |
|
Bristol-Myers Squibb |
1887 |
New York, USA |
- |
- |
- |
- |
- |
- |
|
Amgen Inc. |
1980 |
Thousand Oaks, USA |
- |
- |
- |
- |
- |
- |
USA Pharmaceutical Market Analysis
USA Pharmaceutical Market Growth Drivers:
- Increasing Prevalence of Chronic Diseases: The prevalence of chronic diseases such as cardiovascular disorders, cancer, and diabetes is rising steadily in the USA, driven by ageing populations and lifestyle changes. In 2023, over 37 million Americans suffered from diabetes, while cardiovascular diseases accounted for nearly 697,000 deaths annually, making it the leading cause of death in the country. The World Health Organization reports that non-communicable diseases, including chronic conditions, will continue to dominate healthcare demands. This increasing disease burden boosts the need for pharmaceutical interventions and treatments, accelerating market growth.
- Rising Healthcare Expenditure: The USA leads the world in healthcare spending, with national healthcare expenditures projected to reach around $4.5 trillion in 2024, according to the Centers for Medicare and Medicaid Services (CMS). The country's per capita healthcare spending stood at over $13,000 in 2023. This high expenditure supports the pharmaceutical sector by ensuring robust funding for research, drug development, and innovative treatments, ultimately driving market expansion. With continued governmental and private investments in healthcare infrastructure, the pharmaceutical market is poised for sustained growth.
Source: CMS Healthcare Spending Data - Expansion of Biotechnology Sector: The biotechnology sector in the USA has witnessed rapid expansion, contributing substantially to pharmaceutical innovation. In 2023, the U.S. biotechnology industry saw an investment of around $150 billion in research and development. The focus has been on biologics, gene therapies, and cell-based treatments, which are projected to dominate future pharmaceutical innovations. With biotechnology accounting for nearly 20% of all FDA drug approvals in 2023, the sector's growth is a crucial driver of the pharmaceutical market, facilitating breakthroughs in therapeutic areas like cancer and genetic disorders.
USA Pharmaceutical Market Challenges:
- High Drug Development Costs: Developing a new drug in the USA costs an estimated $2.6 billion, according to recent data from the Tufts Center for the Study of Drug Development. This figure includes the costs of clinical trials, regulatory approvals, and post-marketing studies. The high costs are a substantial burden for pharmaceutical companies, limiting the number of new drugs brought to market. These costs also contribute to rising drug prices, making it challenging for smaller firms to compete.
- Stringent Regulatory Landscape (Clinical Trials, FDA Regulations): The U.S. pharmaceutical market faces substantial regulatory challenges, particularly in the clinical trial and FDA approval process. On average, it takes 10-15 years for a new drug to move from initial discovery to the marketplace, with clinical trials accounting for up to 7 years. Stringent FDA regulations, including safety and efficacy trials, increase the time and cost required for market entry, making the process prohibitive for some companies.
USA Pharmaceutical Market Future Market Outlook
Over the next five years, the USA pharmaceutical market is projected to see substantial growth driven by the rising incidence of chronic diseases, continued innovation in drug discovery, and regulatory support for expedited approvals. Advances in personalized medicine, particularly in the biologics and gene therapy space, will play a major role in reshaping the market. Furthermore, increasing healthcare expenditure and the expanding elderly population are expected to boost demand for pharmaceutical products.
USA Pharmaceutical Market Opportunities:
- Personalized Medicine and Precision Therapeutics: The rise of personalized medicine, which tailors treatment to individual patient profiles based on genetic, environmental, and lifestyle factors, presents a major growth opportunity for the pharmaceutical market. In 2023, personalized medicines accounted for over 40% of new drugs approved by the FDA. The demand for precision therapeutics is expected to grow, particularly in oncology, where targeted treatments are already improving patient outcomes.
- 3.3.2. Increasing Biologics and Biosimilars Adoption: The adoption of biologics and biosimilars is steadily increasing in the USA. In 2023, biologics accounted for around 35% of total pharmaceutical sales. With several high-profile biologics, such as insulin, set to face competition from biosimilars, the market for these products is expanding. Biosimilars offer cost-effective alternatives to expensive biologics, making them increasingly attractive to healthcare providers and patients, thus driving further growth in this segment.
Scope of the Report
|
By Therapeutic Area |
Oncology Cardiovascular Neurology Infectious Diseases Respiratory |
|
By Drug Type |
Prescription Drugs OTC Drugs |
|
By Product Type |
Small Molecule Drugs Biologics Generics Biosimilars |
|
By Distribution Channel |
Hospital Pharmacies Retail Pharmacies Online Pharmacies |
|
By Region |
North South East West |
Products
Key Target Audience Organizations and Entities Who Can Benefit by Subscribing This Report:
Government and Regulatory Bodies
Banks and Financial Institutes
Investors and Venture Capitalists
Pharmaceutical Manufacturers
Biotechnology Companies
Healthcare Companies
Insurance and Reimbursement Agencies and Companies
Time Period Captured in the Report
Historical Period: 2018-2023
Base Year: 2023
Forecast Period: 2023-2028
Table of Contents
1. USA Pharmaceutical Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate
1.4. Key Market Segments (Therapeutics, Prescription & OTC, Biologics, Generics)
2. USA Pharmaceutical Market Size (In USD Bn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis
2.3. Key Market Developments and Milestones
3. USA Pharmaceutical Market Analysis
3.1. Growth Drivers
3.1.1. Increasing Prevalence of Chronic Diseases
3.1.2. Rising Healthcare Expenditure
3.1.3. Government Initiatives and Regulatory Approvals (FDA Approvals)
3.1.4. Expansion of Biotechnology Sector
3.2. Market Challenges
3.2.1. High Drug Development Costs
3.2.2. Stringent Regulatory Landscape (Clinical Trials, FDA Regulations)
3.2.3. Generic Competition and Patent Expirations
3.3. Opportunities
3.3.1. Personalized Medicine and Precision Therapeutics
3.3.2. Increasing Biologics and Biosimilars Adoption
3.3.3. Expanding Geriatric Population
3.4. Trends
3.4.1. Digital Health Integration and Telemedicine
3.4.2. Growing Adoption of AI in Drug Discovery
3.4.3. Advances in Gene Therapy
3.5. Government Regulations
3.5.1. Drug Price Transparency Initiatives (Inflation Reduction Act)
3.5.2. Changes in FDA Drug Review Process
3.5.3. Patent Legislation
3.6. SWOT Analysis (Strengths, Weaknesses, Opportunities, Threats)
3.7. Stakeholder Ecosystem (Pharmaceutical Manufacturers, Contract Research Organizations, Healthcare Providers)
3.8. Porters Five Forces Analysis
3.9. Competition Ecosystem (Patent Strategy, Market Positioning)
4. USA Pharmaceutical Market Segmentation
4.1. By Therapeutic Area (In Value %)
4.1.1. Oncology
4.1.2. Cardiovascular
4.1.3. Neurology
4.1.4. Infectious Diseases
4.1.5. Respiratory
4.2. By Drug Type (In Value %)
4.2.1. Prescription Drugs
4.2.2. Over-the-Counter (OTC) Drugs
4.3. By Product Type (In Value %)
4.3.1. Small Molecule Drugs
4.3.2. Biologics
4.3.3. Generics
4.3.4. Biosimilars
4.4. By Distribution Channel (In Value %)
4.4.1. Hospital Pharmacies
4.4.2. Retail Pharmacies
4.4.3. Online Pharmacies
4.5. By Region (In Value %)
4.5.1. Northeast
4.5.2. Midwest
4.5.3. South
4.5.4. West
5. USA Pharmaceutical Market Competitive Analysis
5.1. Detailed Profiles of Major Companies
5.1.1. Pfizer Inc.
5.1.2. Johnson & Johnson
5.1.3. Merck & Co., Inc.
5.1.4. AbbVie Inc.
5.1.5. Bristol-Myers Squibb
5.1.6. Amgen Inc.
5.1.7. Gilead Sciences
5.1.8. Eli Lilly and Company
5.1.9. Novartis AG
5.1.10. Sanofi
5.1.11. Roche Holding AG
5.1.12. GlaxoSmithKline
5.1.13. AstraZeneca
5.1.14. Teva Pharmaceutical Industries Ltd.
5.1.15. Bayer AG
5.2. Cross Comparison Parameters (Revenue, Market Share, R&D Expenditure, Patent Portfolio, Pipeline Products, Key Therapeutic Focus)
5.3. Market Share Analysis
5.4. Strategic Initiatives (Collaborations, Licensing Agreements, Product Launches)
5.5. Mergers and Acquisitions
5.6. Investment Analysis
5.7. Venture Capital Funding
5.8. Government Grants
5.9. Private Equity Investments
6. USA Pharmaceutical Market Regulatory Framework
6.1. FDA Drug Approval Process
6.2. Compliance Requirements (Good Manufacturing Practices)
6.3. Pricing Controls and Reimbursement Policies
7. USA Pharmaceutical Future Market Size (In USD Bn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth (Precision Medicine, Biologics, Aging Population)
8. USA Pharmaceutical Future Market Segmentation
8.1. By Therapeutic Area (In Value %)
8.2. By Drug Type (In Value %)
8.3. By Product Type (In Value %)
8.4. By Distribution Channel (In Value %)
8.5. By Region (In Value %)
9. USA Pharmaceutical Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis (Total Addressable Market, Serviceable Addressable Market, Serviceable Obtainable Market)
9.2. Customer Cohort Analysis
9.3. Marketing Initiatives
9.4. White Space Opportunity Analysis
Disclaimer Contact UsResearch Methodology
Step 1: Identification of Key Variables
The research process began with constructing an ecosystem map that outlines all major stakeholders in the USA pharmaceutical market. Extensive desk research was conducted using both secondary sources and proprietary databases to capture relevant industry data. This stage focused on identifying the variables that influence market dynamics, such as pricing, regulations, and innovation.
Step 2: Market Analysis and Construction
Next, historical data pertaining to the market size, therapeutic penetration, and revenue generation were compiled and analyzed. This step involved a deep dive into market trends, focusing on drug approvals, market entries, and competitive strategies. Service quality statistics were also evaluated to ensure accurate projections.
Step 3: Hypothesis Validation and Expert Consultation
Key market hypotheses were validated through interviews with industry experts using computer-assisted telephone interviews (CATI). Insights were gathered on critical operational and financial elements, ensuring robust data-backed conclusions.
Step 4: Research Synthesis and Final Output
In the final phase, interactions with pharmaceutical companies allowed for a detailed understanding of market segments, including sales performance and consumer preferences. This ensured that all statistical analyses were validated using a combination of top-down and bottom-up approaches.
Frequently Asked Questions
01. How big is the USA Pharmaceutical Market?
The USA pharmaceutical market is valued at around USD 598s billion, driven by the increasing prevalence of chronic diseases, advanced drug discovery techniques, and substantial R&D investments by key market players.
02. What are the challenges in the USA Pharmaceutical Market?
The USA pharmaceutical market key challenges include high drug development costs, regulatory hurdles such as stringent FDA approval processes, and increasing competition from generics and biosimilars, which drive down profit margins.
03. Who are the major players in the USA Pharmaceutical Market?
The USA pharmaceutical market key players include Pfizer Inc., Johnson & Johnson, Merck & Co., Inc., Bristol-Myers Squibb, and Amgen Inc., who maintain their dominance through a strong pipeline, substantial R&D investments, and numerous FDA-approved products.
04. What are the growth drivers of the USA Pharmaceutical Market?
The USA pharmaceutical market is primarily driven by the rising incidence of chronic diseases, advancements in personalized medicine, and increased healthcare expenditure. In addition, accelerated FDA approvals for breakthrough drugs are a substantial growth driver.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















