
USA Procalcitonin (PCT) Market Outlook to 2030
Region:North America
Author(s):Shambhavi
Product Code:KROD9454
December 2024
95
About the Report
USA Procalcitonin (PCT) Market Overview
- The U.S. Procalcitonin market, as of 2023, demonstrates significant growth in line with increasing cases of sepsis and respiratory infections, which have heightened the demand for PCT tests for early and precise diagnosis. Valued at USD 103 million, this market is propelled by advancements in healthcare diagnostics and the rising adoption of biomarkers for infection control and sepsis management. Large healthcare facilities in metropolitan areas such as New York City, Los Angeles, and Chicago drive demand due to their extensive patient care systems and reliance on advanced diagnostic tools.
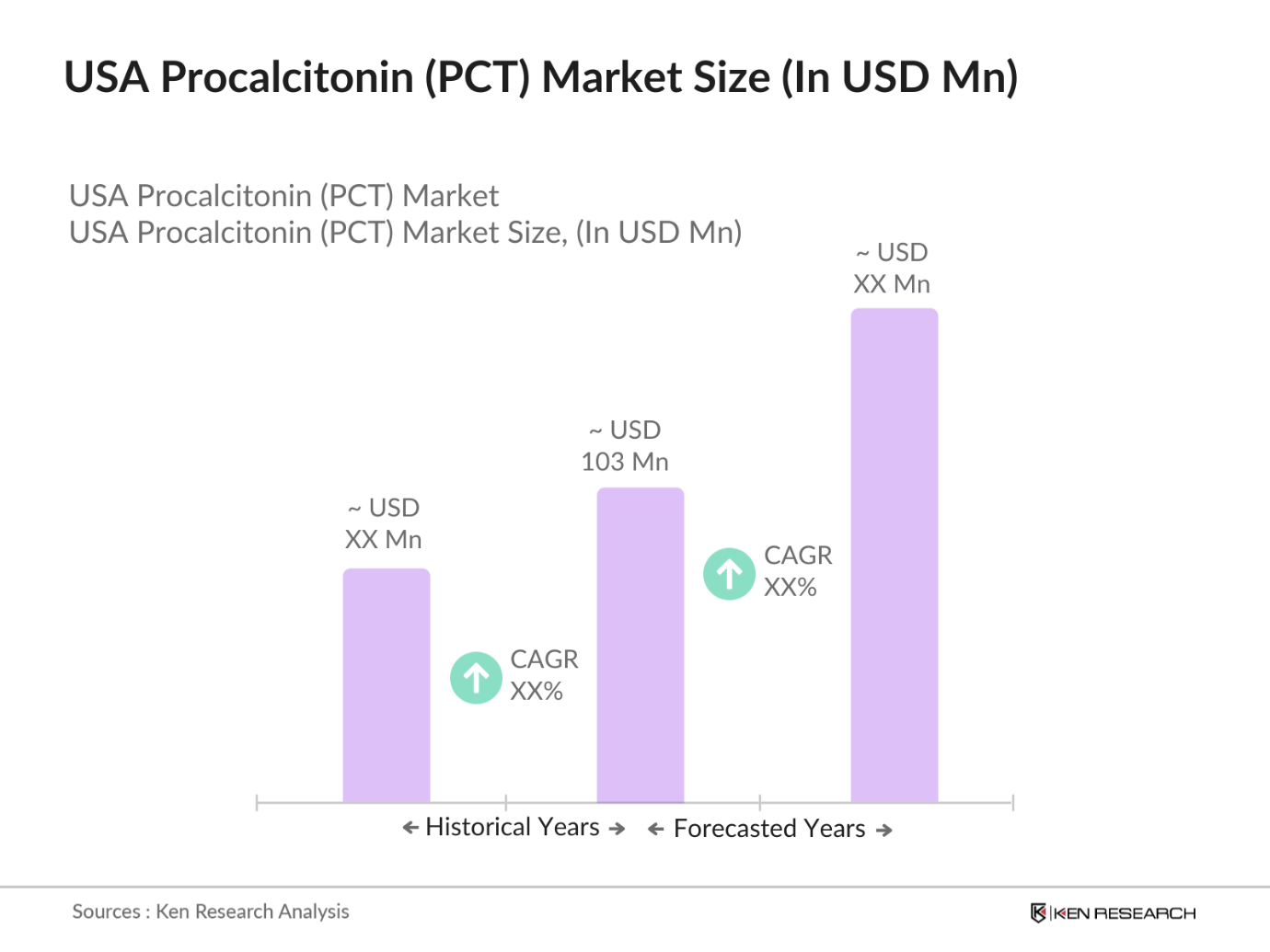
- The dominance of cities like New York and Los Angeles is attributed to their large population, high hospital density, and well-developed healthcare infrastructure. These regions see a high influx of patients requiring urgent care and infection management, making them primary hubs for PCT testing services. Furthermore, the concentration of medical research centers and clinical trials in these areas bolsters the markets growth and adoption of advanced diagnostic technologies.
- The U.S. Food and Drug Administration (FDA) has established specific guidelines for diagnostic testing, including Procalcitonin (PCT) assays. These guidelines ensure that diagnostic tests meet safety and efficacy standards before reaching the market. The FDA's regulatory framework for PCT assays includes premarket approval processes, labeling requirements, and post-market surveillance to monitor the performance of these tests in clinical settings. Adherence to FDA guidelines is crucial for manufacturers to ensure compliance and maintain market access
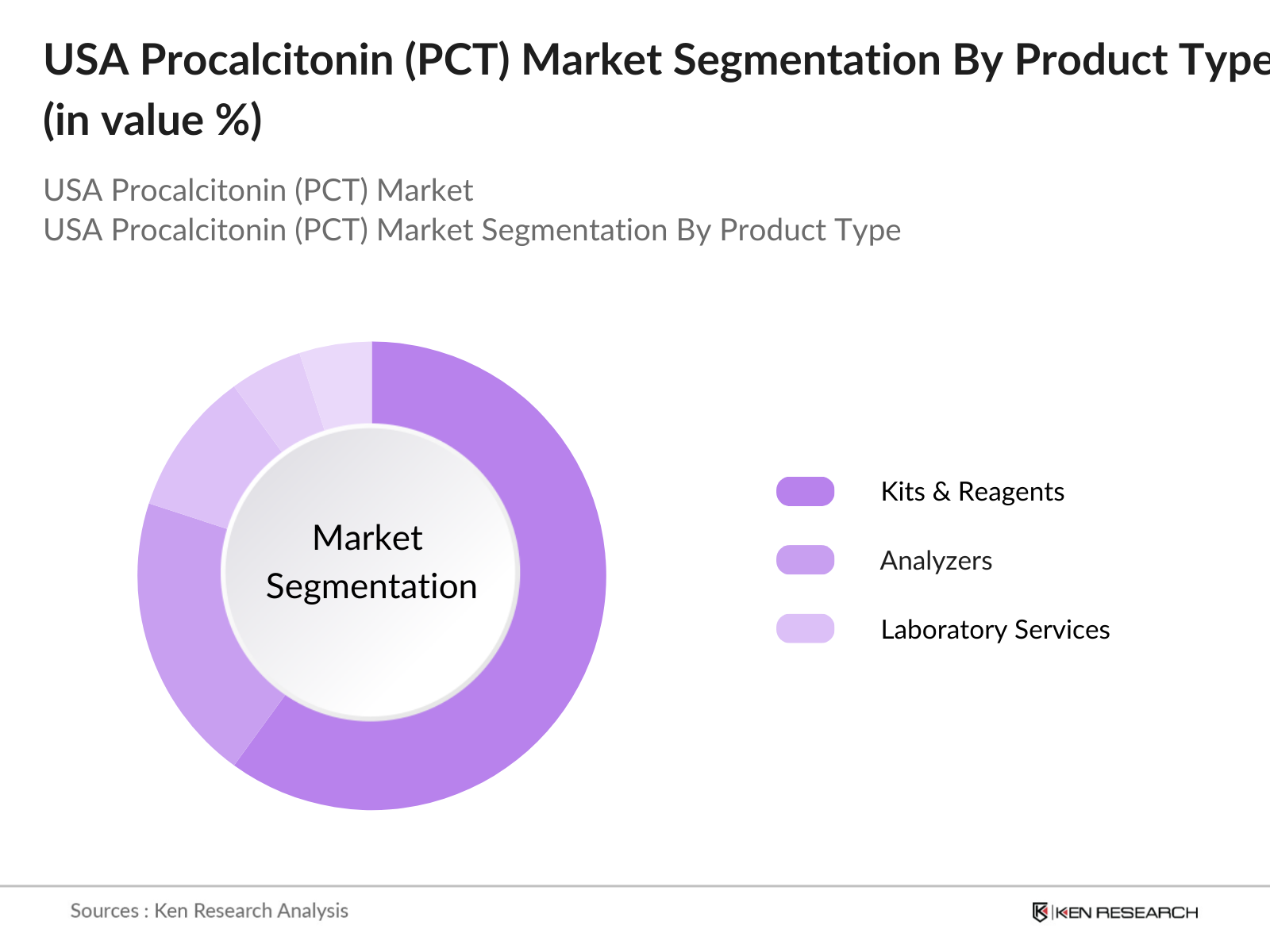
USA Procalcitonin (PCT) Market Segmentation
By Product Type: The Procalcitonin market in the USA is segmented by product type into Kits & Reagents, Analyzers, and Laboratory Services. Kits & Reagents hold the dominant market share due to their frequent use in healthcare facilities for rapid testing and diagnosis. These consumables are critical in emergency scenarios where immediate PCT results can guide treatment decisions, particularly in ICU settings. Hospitals rely on these ready-to-use kits for efficiency and accuracy, driving the demand for this segment.
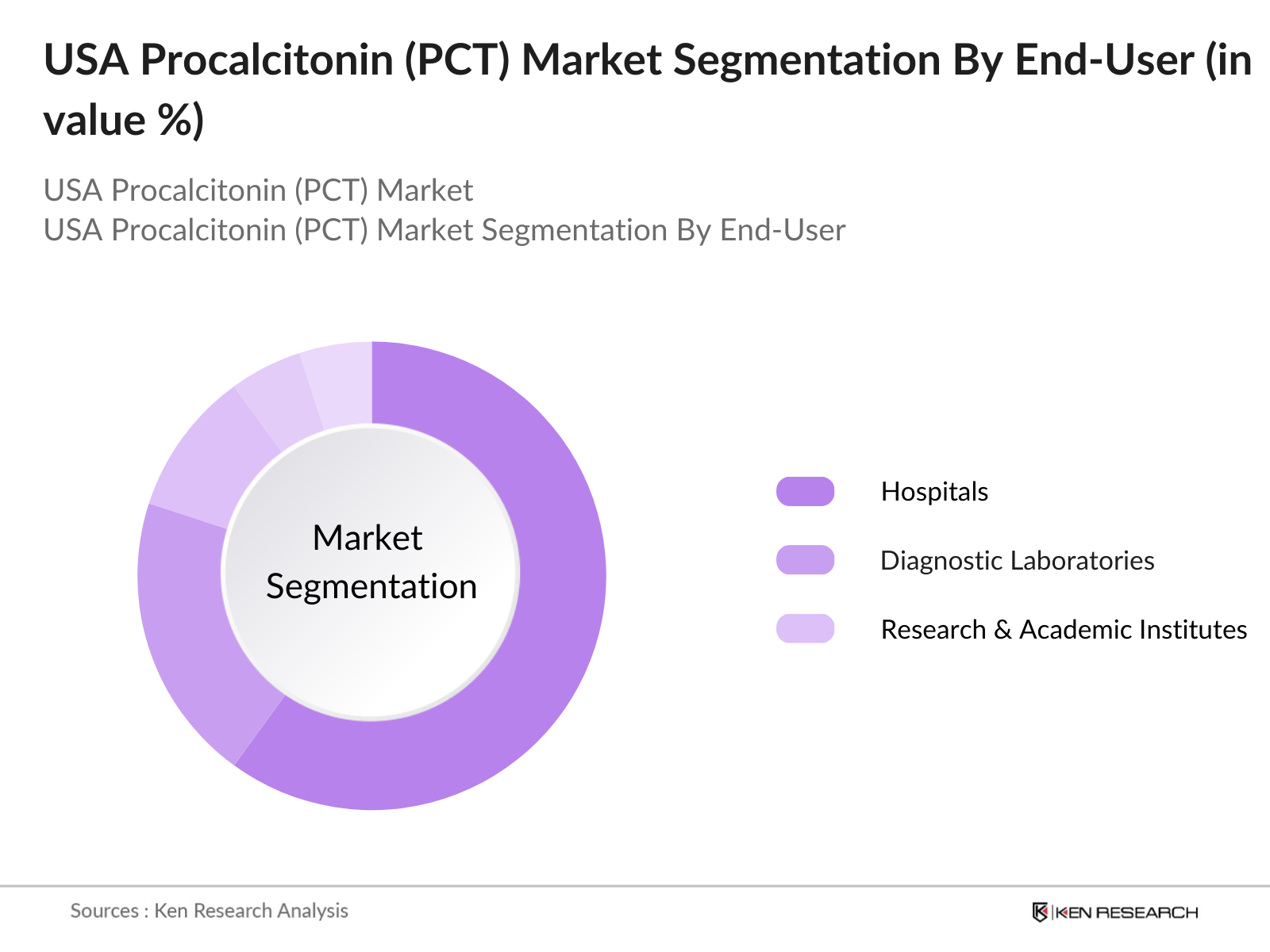
By End User: The market is further segmented by end users, including Hospitals, Diagnostic Laboratories, and Research & Academic Institutes. Hospitals lead this segment due to the high volume of PCT tests conducted in critical care and emergency departments. Procalcitonin testing has become integral to sepsis management protocols within hospitals, contributing to the growth of this sub-segment. Hospital demand is further boosted by increasing patient admissions with infection-related complications, particularly in large hospitals equipped with advanced diagnostic capabilities.
USA Procalcitonin (PCT)Competitive Landscape
The U.S. Procalcitonin market features key players known for their strong product portfolios and extensive distribution networks. Leading companies in the space contribute significantly to market growth through continuous research and development, as well as strategic partnerships.
The competitive landscape is dominated by companies such as Thermo Fisher Scientific and Roche Diagnostics, which benefit from established brand reputations and comprehensive diagnostic product ranges. This consolidation underscores the impact of a few prominent players in the industry.
USA Procalcitonin (PCT) market Analysis
Growth Drivers
- Increasing Incidence of Severe Infections: The rise in severe infections such as sepsis has been a significant driver for the Procalcitonin market. According to the Centers for Disease Control and Prevention (CDC), approximately 1.7 million adults in the United States develop sepsis annually, with a mortality rate of about 20%. This alarming statistic underscores the urgent need for accurate diagnostic tools like Procalcitonin, which help in timely interventions. With rising hospitalizations, the demand for effective diagnostic solutions is expected to surge significantly.
- Rising Demand for Rapid Diagnostic Tools: The healthcare landscape is increasingly leaning towards rapid diagnostics to enhance patient outcomes and streamline treatment pathways. The American Hospital Association reported that hospitals are investing in point-of-care testing solutions that can provide results within hours, directly impacting clinical decisions. As a result, the demand for Procalcitonin tests, which provide rapid insights into bacterial infections, is on the rise, further solidifying its position in the diagnostics market.
- Growing Adoption in Critical Care Settings: The critical care sector is witnessing a shift towards evidence-based practices, with Procalcitonin emerging as a vital biomarker for diagnosing bacterial infections. Studies indicate that the use of PCT-guided protocols in intensive care units (ICUs) leads to reduced antibiotic use and improved patient outcomes. This trend is supported by the Society of Critical Care Medicine, highlighting the increasing integration of Procalcitonin testing in critical care protocols, driving market growth.
Challenges
- High Costs of Procalcitonin Testing: The financial burden associated with Procalcitonin testing can pose a significant challenge for widespread adoption. The cost per test can range from $25 to $150, which may deter smaller healthcare facilities from implementing this technology. In a study by the Healthcare Cost and Utilization Project, high testing costs are cited as a barrier for integrating advanced diagnostic tools in many hospitals, leading to underutilization of Procalcitonin tests in clinical settings.
- Regulatory Hurdles: The Procalcitonin market is subject to rigorous regulatory scrutiny, which can impede timely market entry for new diagnostic products. The Food and Drug Administration (FDA) imposes stringent requirements for clinical validation and safety assessments before any new test can be launched. This regulatory environment can delay innovations and increase development costs, hindering market dynamics and slowing down potential growth in the Procalcitonin sector.
USA Procalcitonin (PCT) Market Future Outlook
Over the next five years, the U.S. Procalcitonin market is projected to experience significant growth. This trend is driven by advancements in diagnostic accuracy, the rising prevalence of sepsis cases, and government initiatives promoting early detection of infections. Additionally, as precision medicine continues to evolve, there is an increasing focus on infection biomarkers like procalcitonin for targeted treatments.
Market Opportunities
- Technological Innovations in Diagnostic Methods: The Procalcitonin market is poised for growth due to ongoing technological advancements that enhance diagnostic capabilities. The integration of automated systems and artificial intelligence (AI) in testing processes not only increases the speed of results but also improves the accuracy of diagnoses. For instance, the development of high-throughput Procalcitonin testing platforms allows laboratories to process larger volumes of tests more efficiently, addressing the increasing demand from healthcare providers
- Increasing Investments in Healthcare Infrastructure: The ongoing investments in healthcare infrastructure across the United States provide significant opportunities for the Procalcitonin market. The American Hospital Association (AHA) reports that U.S. hospitals are projected to invest over $100 billion annually in upgrading facilities and acquiring new technologies, particularly in diagnostic equipment. This investment surge is driven by a need for better infection management and rapid diagnostic solutions.
Scope of the Report
|
Segment |
Sub-Segments |
|
By Test Type |
Automated PCT Testing |
|
Manual PCT Testing |
|
|
By Application |
Respiratory Tract Infections |
|
Sepsis |
|
|
Other Infections |
|
|
By End-User |
Hospitals |
|
Diagnostic Laboratories |
|
|
Home Healthcare |
|
|
By Region |
Northeast |
|
Midwest |
|
|
South |
|
|
West |
Products
Key Target Audience
Investor and Venture Capitalist Firms
Government and Regulatory Bodies (e.g., U.S. Food and Drug Administration)
Hospitals and Diagnostic Centers
Pharmaceutical and Biotech Companies
Clinical Research Organizations (CROs)
Medical Device Distributors
Laboratories and Testing Facilities
Healthcare Providers
Companies
Major Market Players
Thermo Fisher Scientific
Roche Diagnostics
Abbott Laboratories
Siemens Healthineers
bioMrieux
Becton, Dickinson and Company
Beckman Coulter
DiaSorin
Ortho Clinical Diagnostics
Quidel Corporation
Randox Laboratories
Boditech Med Inc.
Bio-Rad Laboratories
Luminex Corporation
Agilent Technologies
Table of Contents
1.USA Procalcitonin (PCT) Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate
1.4. Market Segmentation Overview
2. USA Procalcitonin (PCT) Market Size (In USD Bn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis
2.3. Key Market Developments and Milestones
3. USA Procalcitonin (PCT) Market Analysis
3.1. Growth Drivers
3.1.1. Increasing Incidence of Severe Infections
3.1.2. Rising Demand for Rapid Diagnostic Tools
3.1.3. Growing Adoption in Critical Care Settings
3.1.4. Expansion of Point-of-Care Testing (POCT)
3.2. Market Challenges
3.2.1. High Costs of Procalcitonin Testing
3.2.2. Regulatory Hurdles
3.2.3. Limited Awareness Among Healthcare Professionals
3.3. Opportunities
3.3.1. Technological Innovations in Diagnostic Methods
3.3.2. Increasing Investments in Healthcare Infrastructure
3.3.3. Expansion of Clinical Applications
3.4. Trends
3.4.1. Integration of AI in Diagnostic Testing
3.4.2. Growth of Telehealth Services
3.4.3. Collaborations for Research and Development
3.5. Government Regulation
3.5.1. FDA Guidelines for Diagnostic Testing
3.5.2. Reimbursement Policies
3.5.3. Public Health Initiatives
3.6. SWOT Analysis
3.7. Stake Ecosystem
3.8. Porters Five Forces
3.9. Competition Ecosystem
4. USA Procalcitonin (PCT) Market Segmentation
4.1. By Test Type (In Value %)
4.1.1. Automated PCT Testing
4.1.2. Manual PCT Testing
4.2. By Application (In Value %)
4.2.1. Respiratory Tract Infections
4.2.2. Sepsis
4.2.3. Other Infections
4.3. By End-User (In Value %)
4.3.1. Hospitals
4.3.2. Diagnostic Laboratories
4.3.3. Home Healthcare
4.4. By Region (In Value %)
4.4.1. Northeast
4.4.2. Midwest
4.4.3. South
4.4.4. West
5. USA Procalcitonin (PCT) Market Competitive Analysis
5.1 Detailed Profiles of Major Companies
5.1.1. Roche Diagnostics
5.1.2. Abbott Laboratories
5.1.3. Siemens Healthineers
5.1.4. Thermo Fisher Scientific
5.1.5. Becton, Dickinson and Company
5.1.6. BioMrieux
5.1.7. HiberGene Diagnostics
5.1.8. Karius, Inc.
5.1.9. Sphingotec GmbH
5.1.10. Ortho Clinical Diagnostics
5.1.11. Quidel Corporation
5.1.12. DiaSorin S.p.A.
5.1.13. Grifols S.A.
5.1.14. Genalyte, Inc.
5.1.15. Axon Lab AG
5.2 Cross Comparison Parameters (No. of Employees, Headquarters, Inception Year, Revenue)
5.3. Market Share Analysis
5.4. Strategic Initiatives
5.5. Mergers and Acquisitions
5.6. Investment Analysis
5.7. Venture Capital Funding
5.8. Government Grants
5.9. Private Equity Investments
6. USA Procalcitonin (PCT) Market Regulatory Framework
6.1. Compliance Requirements
6.2. Certification Processes
6.3. Reporting Standards
7. USA Procalcitonin (PCT) Future Market Size (In USD Bn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth
8. USA Procalcitonin (PCT) Future Market Segmentation
8.1. By Test Type (In Value %)
8.2. By Application (In Value %)
8.3. By End-User (In Value %)
8.4. By Region (In Value %)
9. USA Procalcitonin (PCT) Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Customer Cohort Analysis
9.3. Marketing Initiatives
9.4. White Space Opportunity Analysis
Research Methodology
Step 1: Identification of Key Variables
The research begins with identifying all significant stakeholders within the U.S. Procalcitonin Market ecosystem. Key variables influencing market dynamics, such as usage trends in healthcare settings, product type demand, and regulatory influences, are determined through comprehensive desk research.
Step 2: Market Analysis and Construction
Historical market data from reliable industry sources, including revenue growth and usage statistics, is compiled to construct the market landscape. Market segmentation data further aids in understanding the demand patterns and growth potential for each sub-segment.
Step 3: Hypothesis Validation and Expert Consultation
Expert interviews are conducted with healthcare professionals and industry experts to validate market assumptions. These discussions offer insights into practical PCT applications, hospital procurement patterns, and evolving industry demands.
Step 4: Research Synthesis and Final Output
Finally, data synthesis is conducted through quantitative modeling to generate a cohesive market report. The analysis is refined using expert insights to provide an accurate and actionable understanding of the U.S. Procalcitonin market.
Frequently Asked Questions
01. How big is the U.S. Procalcitonin Market?
The U.S. Procalcitonin market is valued at approximately USD 103 million, driven by the increasing need for rapid diagnostic solutions in infection management and sepsis care.
02. What are the challenges in the U.S. Procalcitonin Market?
Challenges include high costs of advanced diagnostic tests, the need for skilled professionals to interpret results, and stringent regulatory requirements for diagnostic approvals.
03. Who are the major players in the U.S. Procalcitonin Market?
Key players include Thermo Fisher Scientific, Roche Diagnostics, and Siemens Healthineers, which dominate due to their extensive diagnostic product lines, distribution networks, and established industry presence.
04. What factors are driving the U.S. Procalcitonin Market?
The market is driven by rising infection rates, particularly sepsis, the importance of early diagnosis, and increased adoption of PCT tests in critical care units to guide treatment decisions.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















