
KSA In Vitro Diagnostics Market Outlook to 2030
Region:Middle East
Author(s):Sanjeev
Product Code:KROD3827
October 2024
91
About the Report
KSA In Vitro Diagnostics Market Overview
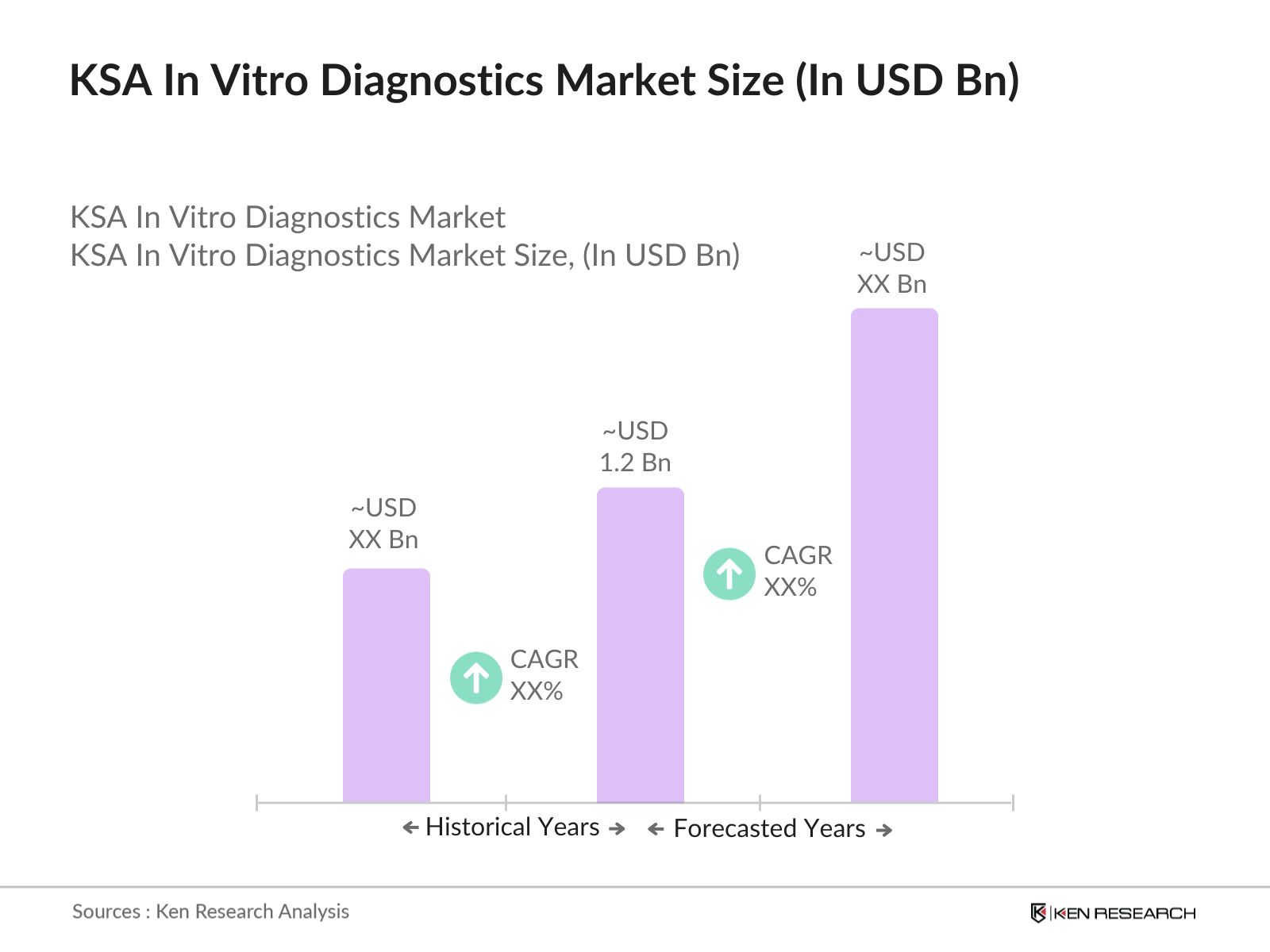
- The KSA In Vitro Diagnostics market is valued at USD 1.2 billion, driven by factors such as rising healthcare expenditure, increased prevalence of chronic diseases, and advancements in diagnostic technologies. The growth is further supported by government initiatives aimed at improving healthcare infrastructure and encouraging local manufacturing. The market's development reflects a robust healthcare landscape where innovation and accessibility are prioritized, aligning with global health standards.
- Dominant cities in the KSA market include Riyadh and Jeddah, which serve as healthcare hubs with advanced medical facilities and research institutions. These cities are characterized by a high concentration of hospitals and diagnostic laboratories, fostering an environment conducive to the growth of in vitro diagnostic services. The support from the government, coupled with investments in healthcare technology, positions these cities at the forefront of the market.
- The KSA government has implemented national health policies that promote the use of in vitro diagnostics to improve public health outcomes. As part of Vision 2030, the Ministry of Health aims to enhance diagnostic capabilities through policy initiatives that encourage innovation and access to advanced technologies. In 2023, healthcare investments linked to these policies totaled SAR 12 billion, reinforcing the governments commitment to improving healthcare infrastructure and services.
KSA In Vitro Diagnostics Market Segmentation
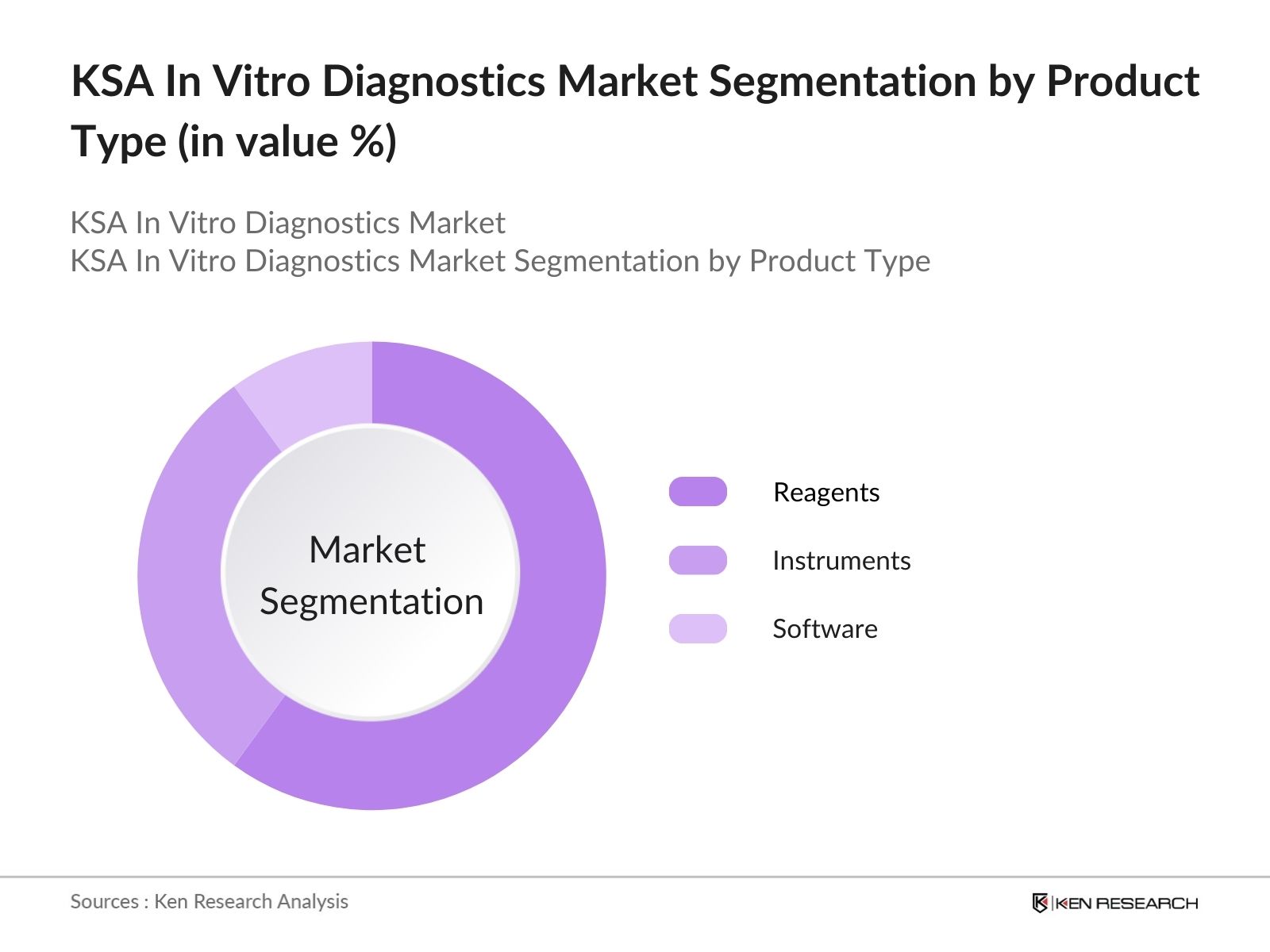
- By Product Type: The market is segmented by product type into reagents, instruments, and software. Among these, reagents hold a dominant market share due to their critical role in the accuracy and efficiency of diagnostic testing. The demand for reagents is bolstered by the increasing volume of diagnostic tests being conducted across various healthcare settings. As healthcare providers focus on delivering accurate results promptly, the reliance on high-quality reagents continues to rise, making them indispensable in the diagnostic process.
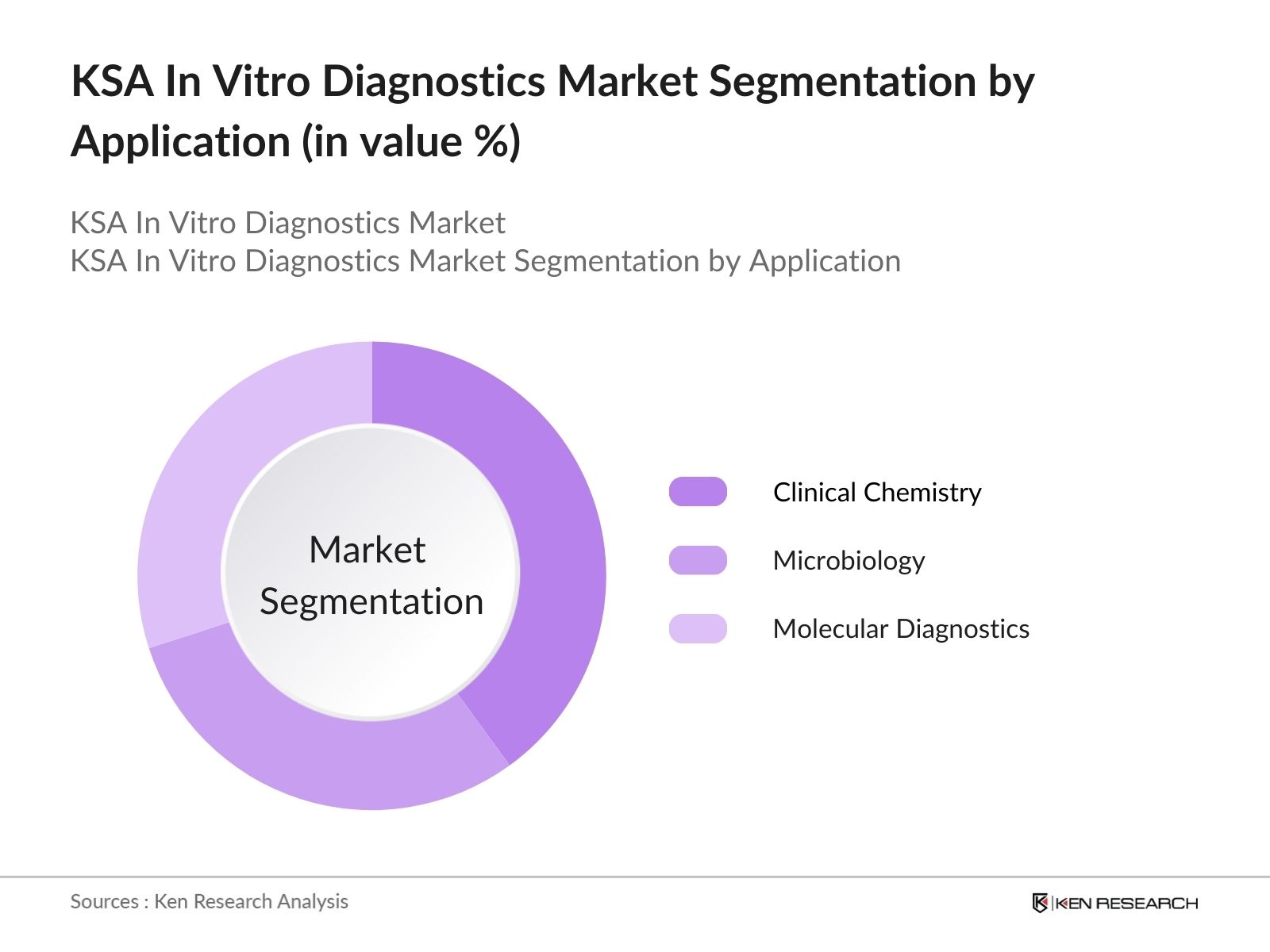
- By Application: The market is also segmented by application, including clinical chemistry, microbiology, and molecular diagnostics. Molecular diagnostics has emerged as the leading segment, driven by its pivotal role in personalized medicine and infectious disease detection. The increasing prevalence of genetic disorders and the growing focus on targeted therapies have led to a surge in demand for molecular diagnostic tests. This segments growth is supported by technological advancements that enhance the sensitivity and specificity of testing methods.
KSA In Vitro Diagnostics Market Competitive Landscape
The KSA In Vitro Diagnostics market is characterized by the presence of several major players, including global leaders and local manufacturers. Key players include Roche Diagnostics, Abbott Laboratories, and Siemens Healthineers. The market dynamics reflect a competitive landscape where innovation, product portfolio diversification, and strategic collaborations are essential for sustaining market presence. The consolidation of key players indicates their significant influence on market trends and consumer preferences.
|
Company |
Establishment Year |
Headquarters |
Parameter 1 |
Parameter 2 |
Parameter 3 |
Parameter 4 |
Parameter 5 |
Parameter 6 |
|
Roche Diagnostics |
1896 |
Basel, Switzerland |
||||||
|
Abbott Laboratories |
1888 |
Abbott Park, USA |
||||||
|
Siemens Healthineers |
1847 |
Erlangen, Germany |
||||||
|
Thermo Fisher Scientific |
1956 |
Waltham, USA |
||||||
|
BD (Becton, Dickinson and Co.) |
1897 |
Franklin Lakes, USA |
KSA In Vitro Diagnostics Industry Analysis
Growth Drivers
- Rising Incidence of Chronic Diseases: The Kingdom of Saudi Arabia (KSA) has experienced a significant increase in chronic diseases, with diabetes prevalence rising to 18.3% in 2022. The growing burden of conditions such as diabetes, cardiovascular diseases, and respiratory disorders necessitates advanced diagnostic tools. In 2023, the healthcare expenditure in KSA reached approximately SAR 191 billion, indicating a commitment to improving health services, including diagnostic capabilities. As chronic diseases become more prevalent, the demand for in vitro diagnostic tests is expected to grow, driving innovations in diagnostics and healthcare infrastructure.
- Technological Advancements in Diagnostic Tools: Technological advancements are revolutionizing the in vitro diagnostics sector in KSA. The adoption of innovative diagnostic technologies, including PCR and next-generation sequencing, is expanding. In 2023, investments in healthcare technology in KSA amounted to SAR 10 billion, highlighting the focus on enhancing diagnostic accuracy and efficiency. These innovations not only improve patient outcomes but also increase the speed of disease detection, thereby supporting the growing demand for diagnostic testing in various healthcare settings.
- Growing Geriatric Population: KSA's geriatric population is expected to rise significantly, with projections indicating that the number of individuals aged 60 and above will reach 3.5 million by 2025. This demographic shift is accompanied by an increase in age-related diseases, further driving the need for effective diagnostic solutions. The healthcare system's focus on providing specialized diagnostic services for the elderly underscores the importance of in vitro diagnostics in managing chronic conditions prevalent among this population segment.
Market Challenges
- Regulatory Compliance and Approval Processes: The regulatory landscape for in vitro diagnostics in KSA poses challenges due to stringent compliance requirements. The approval process for new diagnostic tests can be lengthy, with an average time of 18 months for registration. This delay can hinder timely access to innovative diagnostic solutions. As the market evolves, it is crucial for companies to navigate these regulatory hurdles effectively to ensure that they can bring their products to market swiftly and meet the growing demand for diagnostics.
- High Initial Costs of Diagnostic Equipment: The initial costs associated with in vitro diagnostic equipment remain a significant barrier in KSA, with expenditures often exceeding SAR 1 million for advanced systems. These high costs can limit accessibility, especially in public healthcare facilities, which may struggle to secure funding. As a result, hospitals may delay the adoption of necessary diagnostic technologies, impacting patient care and diagnostic turnaround times.
KSA In Vitro Diagnostics Market Future Outlook
Over the next five years, the KSA In Vitro Diagnostics market is expected to experience substantial growth, driven by continuous government support, technological innovations, and an increasing focus on preventive healthcare. The market will likely benefit from expanding healthcare access and the rising demand for accurate and rapid diagnostic solutions, which are essential for effective patient management and treatment outcomes.
Future Market Opportunities
- Expansion of Point-of-Care Testing: The demand for point-of-care testing (POCT) is growing in KSA, driven by the need for rapid diagnostics. In 2023, the number of POCT devices in use increased by 25%, reflecting a shift towards more accessible testing solutions in hospitals and clinics. This trend is supported by the government's efforts to enhance healthcare delivery in remote areas, providing significant growth opportunities for manufacturers of portable diagnostic devices.
- Advancements in Molecular Diagnostics: Molecular diagnostics are gaining traction in KSA, with market adoption expected to increase as healthcare providers recognize their potential. The introduction of advanced molecular tests has led to improved accuracy in detecting infectious diseases, particularly in response to COVID-19. In 2023, molecular diagnostics accounted for approximately SAR 4 billion in healthcare spending, showcasing a robust opportunity for growth in this segment.
Scope of the Report
|
By Application |
Clinical Chemistry Microbiology Molecular Diagnostics |
|
By Product Type |
Reagents Instruments Software |
|
By Deployment Mode |
On-Premises Cloud-Based |
|
By End-User Industry |
BFSI Government and Public Sector Healthcare IT and Telecommunications Retail and E-Commerce |
|
By Region |
North East West South |
Products
Key Target Audience
Hospitals and Healthcare Providers
Diagnostic Laboratories
Research Institutions
Investments and Venture Capitalist Firms
Government and Regulatory Bodies (Ministry of Health)
Pharmaceutical Companies
Medical Device Manufacturers
Health Insurance Companies
Companies
Major Players
-
Roche Diagnostics
Abbott Laboratories
Siemens Healthineers
Thermo Fisher Scientific
BD (Becton, Dickinson and Co.)
Bio-Rad Laboratories
Hologic Inc.
QIAGEN
Agilent Technologies
PerkinElmer Inc.
Sysmex Corporation
Danaher Corporation
Ortho Clinical Diagnostics
Abcam
Enzo Biochem
Table of Contents
1. KSA In Vitro Diagnostics Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate
1.4. Market Segmentation Overview
2. KSA In Vitro Diagnostics Market Size (In USD Bn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis
2.3. Key Market Developments and Milestones
3. KSA In Vitro Diagnostics Market Analysis
3.1. Growth Drivers
3.1.1. Rising Incidence of Chronic Diseases
3.1.2. Technological Advancements in Diagnostic Tools
3.1.3. Government Initiatives and Funding
3.1.4. Growing Geriatric Population
3.2. Market Challenges
3.2.1. Regulatory Compliance and Approval Processes
3.2.2. High Initial Costs of Diagnostic Equipment
3.2.3. Limited Awareness in Emerging Regions
3.3. Opportunities
3.3.1. Expansion of Point-of-Care Testing
3.3.2. Advancements in Molecular Diagnostics
3.3.3. Growth in Home Healthcare Services
3.4. Trends
3.4.1. Integration of AI and Machine Learning
3.4.2. Shift Towards Personalized Medicine
3.4.3. Increasing Demand for Rapid Diagnostic Tests
3.5. Government Regulation
3.5.1. National Health Policies
3.5.2. Quality Assurance Programs
3.5.3. Pricing and Reimbursement Policies
3.6. SWOT Analysis
3.7. Stake Ecosystem
3.8. Porters Five Forces
3.9. Competition Ecosystem
4. KSA In Vitro Diagnostics Market Segmentation
4.1. By Product Type (In Value %)
4.1.1. Reagents
4.1.2. Instruments
4.1.3. Software
4.2. By Application (In Value %)
4.2.1. Clinical Chemistry
4.2.2. Microbiology
4.2.3. Molecular Diagnostics
4.3. By End-User (In Value %)
4.3.1. Hospitals
4.3.2. Diagnostic Laboratories
4.3.3. Research Institutions
4.4. By Region (In Value %)
4.4.1. Riyadh
4.4.2. Jeddah
4.4.3. Dammam
4.5. By Technology (In Value %)
4.5.1. ELISA
4.5.2. PCR
4.5.3. Next-Generation Sequencing
5. KSA In Vitro Diagnostics Market Competitive Analysis
5.1. Detailed Profiles of Major Companies
5.1.1. Roche Diagnostics
5.1.2. Abbott Laboratories
5.1.3. Siemens Healthineers
5.1.4. Thermo Fisher Scientific
5.1.5. BD (Becton, Dickinson and Company)
5.1.6. Bio-Rad Laboratories
5.1.7. Hologic Inc.
5.1.8. QIAGEN
5.1.9. Agilent Technologies
5.1.10. PerkinElmer Inc.
5.1.11. Sysmex Corporation
5.1.12. Danaher Corporation
5.1.13. Ortho Clinical Diagnostics
5.1.14. Abcam
5.1.15. Enzo Biochem
5.2. Cross Comparison Parameters (No. of Employees, Headquarters, Inception Year, Revenue, Product Portfolio, Market Presence, Strategic Alliances, R&D Investment)
5.3. Market Share Analysis
5.4. Strategic Initiatives
5.5. Mergers And Acquisitions
5.6. Investment Analysis
5.7. Venture Capital Funding
5.8. Government Grants
5.9. Private Equity Investments
6. KSA In Vitro Diagnostics Market Regulatory Framework
6.1. Regulatory Bodies
6.2. Compliance Requirements
6.3. Certification Processes
7. KSA In Vitro Diagnostics Future Market Size (In USD Bn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth
8. KSA In Vitro Diagnostics Future Market Segmentation
8.1. By Product Type (In Value %)
8.2. By Application (In Value %)
8.3. By End-User (In Value %)
8.4. By Region (In Value %)
8.5. By Technology (In Value %)
9. KSA In Vitro Diagnostics Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Customer Cohort Analysis
9.3. Marketing Initiatives
9.4. White Space Opportunity Analysis
Research Methodology
Step 1: Identification of Key Variables
The initial phase involves creating a comprehensive ecosystem map that includes all major stakeholders within the KSA In Vitro Diagnostics market. This is supported by extensive desk research using secondary and proprietary databases to gather relevant industry-level information. The goal is to identify and define critical variables that impact market dynamics.
Step 2: Market Analysis and Construction
This phase entails compiling and analyzing historical data related to the KSA In Vitro Diagnostics market. We assess market penetration rates, the ratio of diagnostic services to healthcare providers, and corresponding revenue generation. A review of service quality metrics will ensure the reliability and accuracy of revenue estimates.
Step 3: Hypothesis Validation and Expert Consultation
Market hypotheses will be formulated and validated through consultations with industry experts via computer-assisted telephone interviews (CATI). These consultations encompass a range of stakeholders, providing valuable operational and financial insights that will enhance the accuracy of the market data.
Step 4: Research Synthesis and Final Output
The final phase includes engaging with multiple in vitro diagnostic manufacturers to gather in-depth insights on product segments, sales performance, and consumer preferences. This engagement will help corroborate and refine statistics derived from the bottom-up approach, ensuring a comprehensive and validated analysis of the KSA In Vitro Diagnostics market.
Frequently Asked Questions
1 How big is the KSA In Vitro Diagnostics Market?
The KSA In Vitro Diagnostics market is valued at USD 1.2 billion, driven by rising healthcare expenditure, increased prevalence of chronic diseases, and advancements in diagnostic technologies.
2 What are the challenges in the KSA In Vitro Diagnostics Market?
Challenges include regulatory compliance, high initial costs of diagnostic equipment, and limited awareness in emerging regions. These factors may hinder the market's potential growth.
3 Who are the major players in the KSA In Vitro Diagnostics Market?
Key players include Roche Diagnostics, Abbott Laboratories, and Siemens Healthineers, among others. These companies dominate due to their extensive product portfolios and strong market presence.
4 What are the growth drivers of the KSA In Vitro Diagnostics Market?
Growth drivers include government initiatives to improve healthcare infrastructure, technological advancements in diagnostic tools, and the increasing incidence of chronic diseases in the population.
5 What are the future prospects of the KSA In Vitro Diagnostics Market?
The market is expected to witness significant growth driven by continuous government support, advancements in diagnostic technology, and rising consumer demand for effective healthcare solutions.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















