
KSA Molecular Diagnostics Market Outlook to 2030
Region:Middle East
Author(s):Shubham Kashyap
Product Code:KROD3288
November 2024
83
About the Report
KSA Molecular Diagnostics Market Overview
- The KSA molecular diagnostics market has experienced rapid growth in recent years reaching a market size of USD 530 Mn, driven by increasing healthcare expenditures, advancements in molecular testing technologies, and a growing demand for personalized medicine. The market has also benefited from an increased focus on infectious disease diagnostics, particularly following the COVID-19 pandemic, which heightened the need for advanced diagnostic tools.
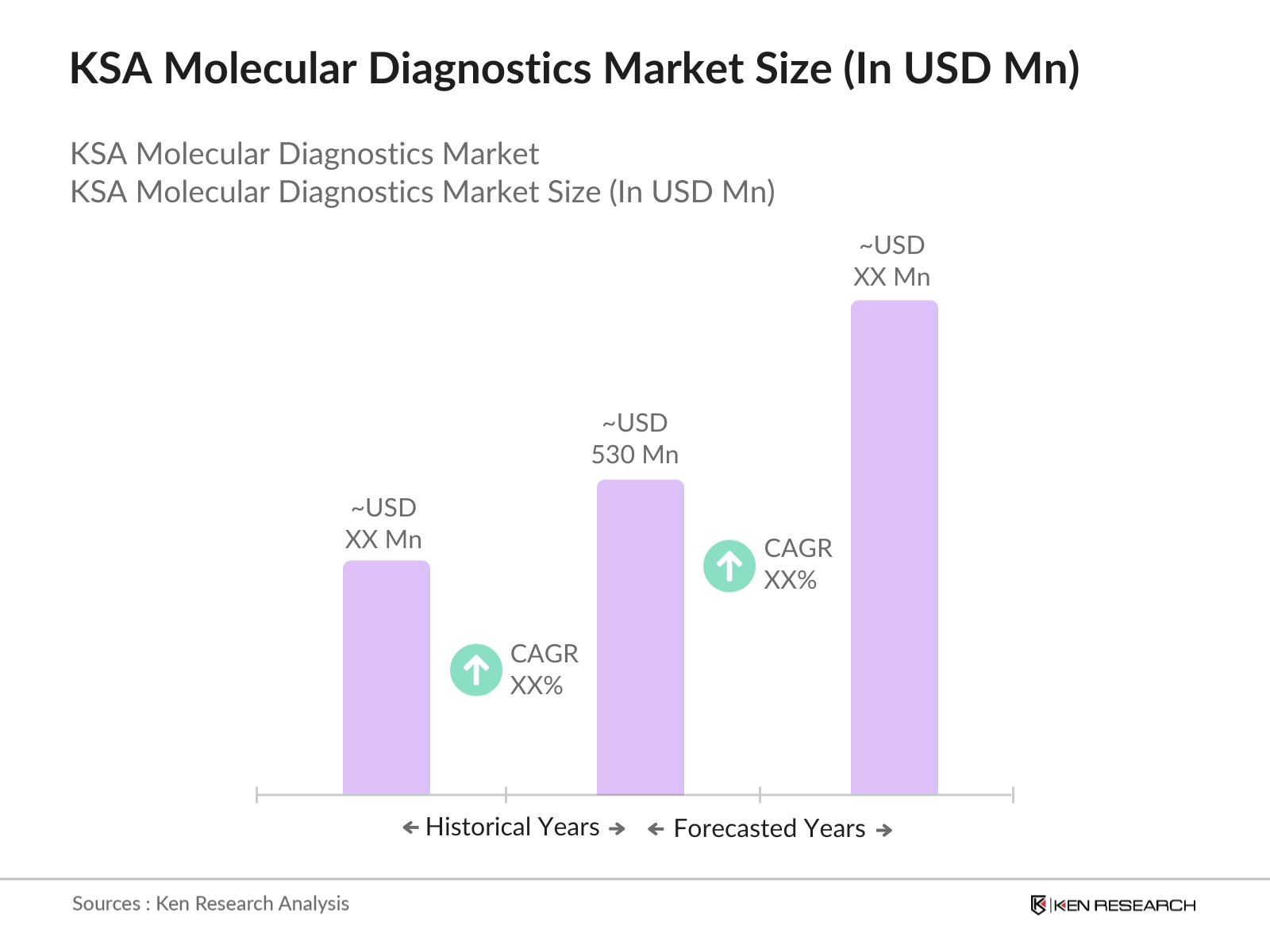
- Major urban centers like Riyadh, Jeddah, and Dammam serve as key hubs for molecular diagnostic services, with numerous healthcare facilities adopting state-of-the-art testing methods to improve patient outcomes. Additionally, the government's Vision 2030 initiative, aimed at transforming the healthcare sector, plays a crucial role in the markets expansion by promoting modern healthcare infrastructure and boosting investment in cutting-edge medical technologies.
- The Saudi Food and Drug Authority (SFDA) plays a critical role in regulating molecular diagnostics to ensure safety, efficacy, and compliance with international standards. The SFDA mandates that diagnostic equipment, including molecular diagnostics technologies such as PCR and NGS, meet stringent quality standards such as ISO 13485 for medical devices and ISO 15189 for medical laboratories.
KSA Molecular Diagnostics Market Segmentation
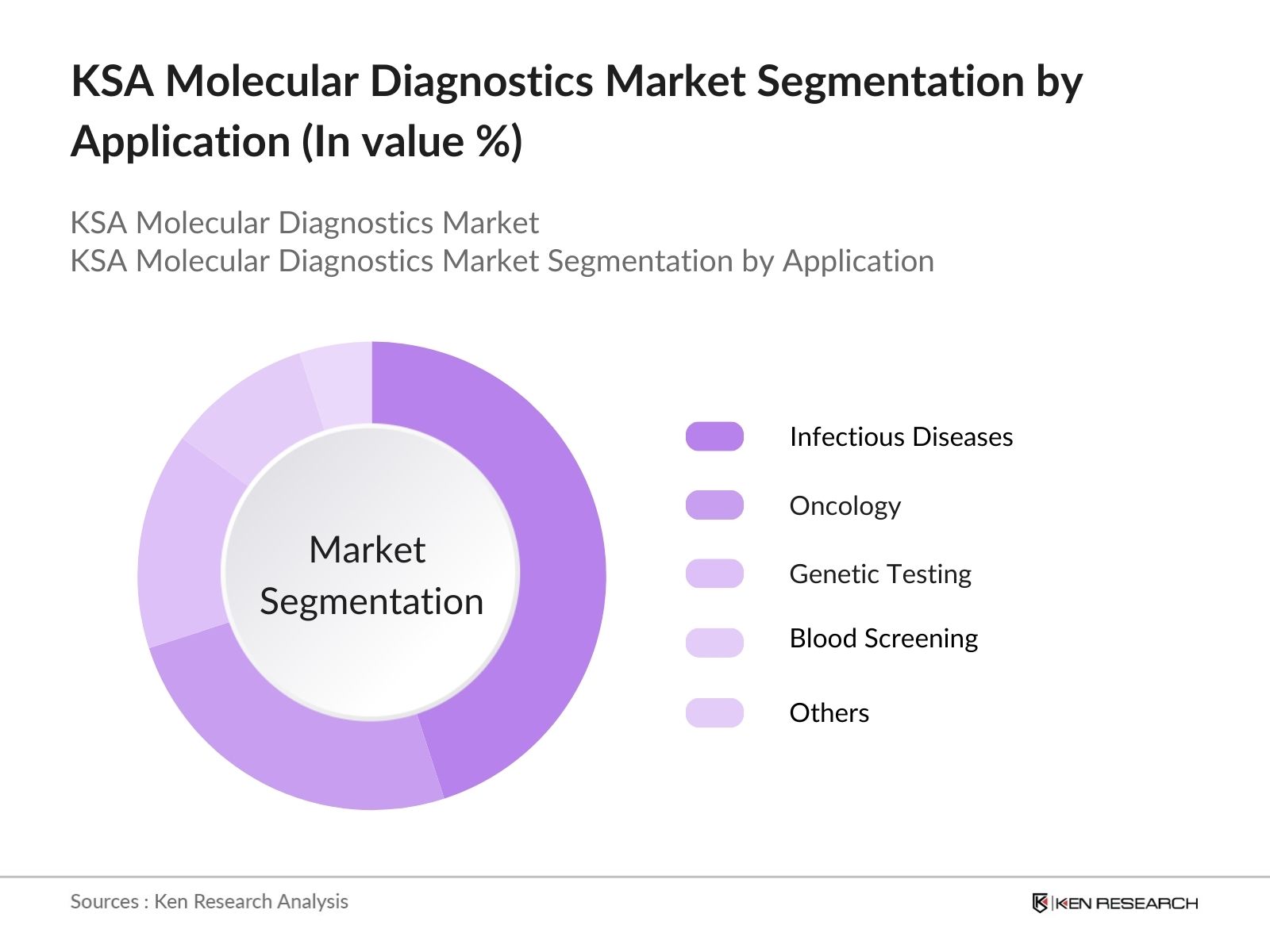
- By Application: The market is segmented by application into infectious diseases, oncology, genetic testing, blood screening and others. Infectious diseases hold the largest market share, driven by the need for accurate and rapid testing for pathogens like SARS-CoV-2, MERS-CoV, and other emerging viruses. Oncology is also witnessing rapid growth due to the increasing prevalence of cancer and the rising use of molecular diagnostics for early detection and personalized treatment.
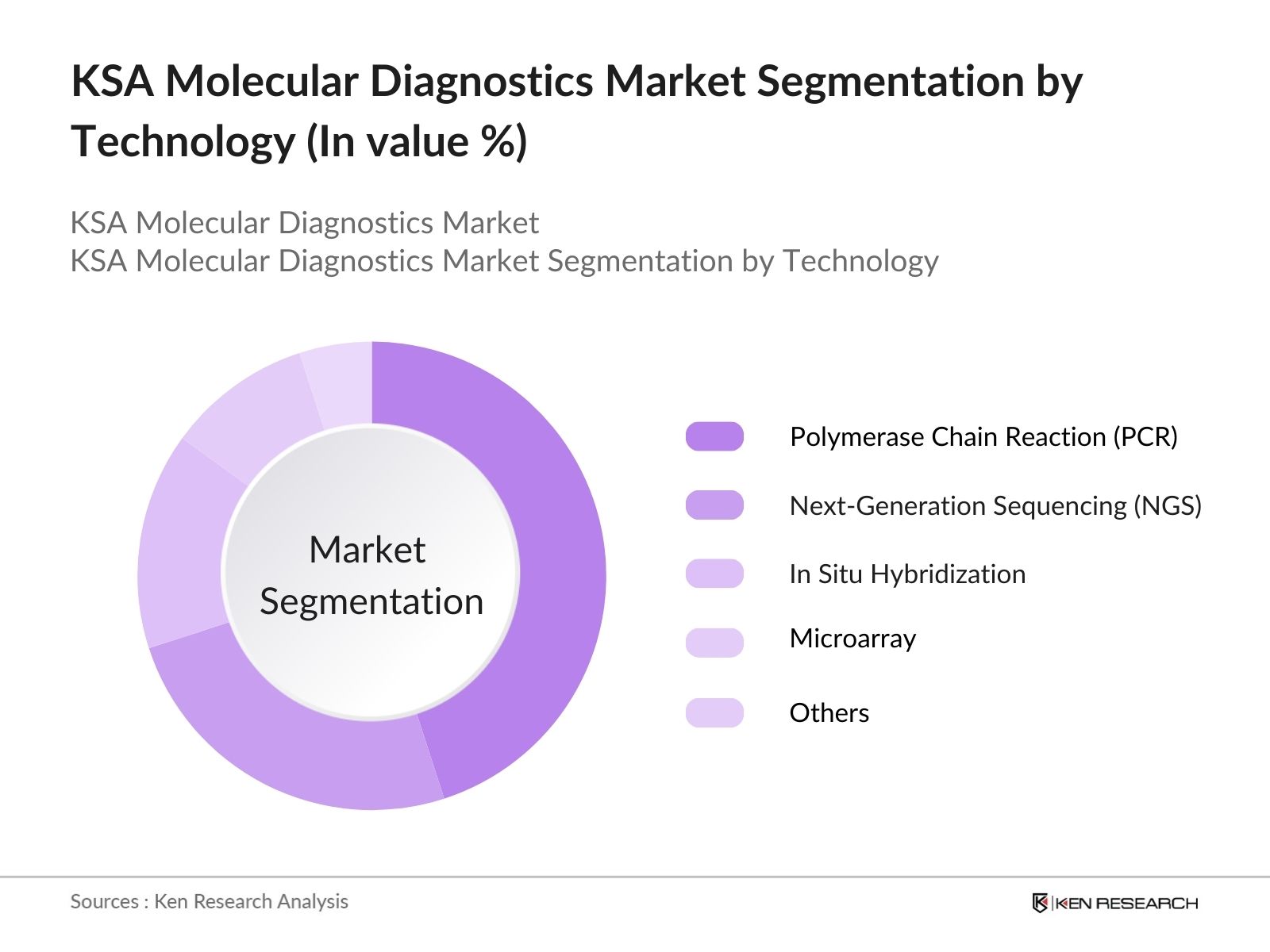
- By Technology: The market is also segmented by technology into Polymerase Chain Reaction (PCR), Next-Generation Sequencing (NGS), In Situ Hybridization, and Microarray. PCR remains the most dominant technology, accounting for the largest market share due to its widespread use in detecting infectious diseases and its established presence in healthcare facilities. The ease of use, cost-effectiveness, and speed of PCR tests have cemented its place as the leading technology in the market.
KSA Molecular Diagnostics Market Competitive Landscape
The KSA molecular diagnostics market is competitive, with a blend of international players and local companies providing advanced diagnostic solutions. Major companies such as Roche Diagnostics, Abbott Laboratories, and Thermo Fisher Scientific are key market players, offering a wide array of molecular diagnostic tests and instruments. These companies are at the forefront of technological advancements and have established partnerships with local healthcare providers to ensure their products meet the regions healthcare needs.
|
Company Name |
Establishment Year |
Headquarters |
Revenue (2023) |
Employees |
Key Product |
R&D Investment |
Key Clients |
Partnerships |
|
Roche Diagnostics |
1896 |
Switzerland |
||||||
|
Abbott Laboratories |
1888 |
USA |
||||||
|
Thermo Fisher Scientific |
1956 |
USA |
||||||
|
Al Borg Diagnostics |
1999 |
Saudi Arabia |
||||||
|
Almana General Hospital |
1949 |
Saudi Arabia |
KSA Molecular Diagnostics Industry Analysis
Growth Drivers:
- Rising Prevalence of Infectious Diseases: Infectious diseases like COVID-19, MERS, and Tuberculosis continue to challenge the Kingdom of Saudi Arabia (KSA). In 2024, Saudi Arabia reported over 841,469 confirmed cases of COVID-19, with around 9646 deaths. Tuberculosis also remains prevalent, with 3,000 cases reported annually in the country. These figures underscore the demand for advanced molecular diagnostics in tracking and managing infectious diseases. The MERS-CoV virus, which caused 2,613 cases and 941 deaths globally, including cases in Saudi Arabia, further drives the need for robust diagnostics technology in the regionchnological advancements.
- Technological Advancements in Next-Generation Sequencing, PCR and AI in Diagnostics: Technological innovations such as Next-Generation Sequencing (NGS), PCR, and AI-based diagnostics are revolutionizing molecular diagnostics in KSA. Saudi Arabia has invested heavily in NGS-based genomic research, with the King Faisal Specialist Hospital (KFSH) performing over 3,000 genomic sequencing tests annually. Real-Time PCR remains central to diagnosing infectious diseases and is widely used across 50+ public and private laboratories in Saudi Arabia. AI is gaining traction in diagnostic applications, including cancer diagnostics, as part of the government's investment in smart healthcare systems.
- Increasing Focus on Precision Medicine: Saudi Arabia is increasingly focusing on precision medicine, especially in oncology diagnostics. In 2024, cancer was responsible for nearly 13,339 deaths annually in KSA, with breast and colorectal cancers being the most prevalent. Genetic testing, such as BRCA gene testing for breast cancer, is becoming critical for early diagnosis and treatment plans. Vision 2030 includes substantial investments to enhance oncology services, expanding diagnostic capabilities in precision medicine. Currently, around 30% of hospitals in the country offer some form of genetic testing for cancer.
Market Challenges:
- High Costs of Molecular Diagnostic Technologies: The cost of molecular diagnostic technologies, such as PCR and NGS, remains a significant barrier in Saudi Arabia. These technologies require advanced equipment and consumables, which are costly and difficult for smaller hospitals and clinics to afford. NGS machines and PCR equipment are especially expensive, limiting widespread adoption of lower-budget healthcare facilities. Although the government has made efforts to support the healthcare sector, only a portion of the healthcare budget is allocated to advanced diagnostic tools, which creates challenges for institutions looking to implement cutting-edge molecular diagnostic technologies.
- Shortage of Skilled Workforce: Despite advancements in molecular diagnostics, a significant shortage of skilled professionals remains in Saudi Arabia. The country lacks an adequate number of trained laboratory technicians and genetic counselors who are critical to the functioning of molecular diagnostic labs. This gap affects the overall capacity of healthcare facilities to offer advanced diagnostic services. The Ministry of Health has recognized this issue and initiated programs to address the shortage through training and education, but the demand for professionals still outpaces the available workforce, posing a challenge to the sector's growth and effectiveness.
KSA Molecular Diagnostics Market Future Outlook
The KSA molecular diagnostics market is expected to experience continued growth over the forecast period, supported by increasing investments in healthcare infrastructure, the rising prevalence of infectious diseases, and technological advancements in diagnostics. The Saudi government's commitment to enhancing healthcare under Vision 2030 will play a critical role in driving market expansion.
Future Market Opportunities:
- Expansion of Genetic Testing Services: Genetic testing services are rapidly expanding in Saudi Arabia, particularly in cancer genomics and pre-natal screening. With nearly 10,000 cancer deaths annually, the demand for genomic testing is growing. Hospitals such as the King Abdullah International Medical Research Center are offering expanded services, performing over 5,000 genomic tests yearly. Pre-natal screening is also gaining momentum, especially with government initiatives promoting maternal healthcare, resulting in over 150,000 prenatal screenings in 2023. The rise in demand offers significant opportunities for further market expansion.
- Growth in Point-of-Care Diagnostics: Point-of-care diagnostics (PoC) is becoming increasingly popular in KSA, particularly for infectious diseases. Portable PCR devices, capable of providing results in under an hour, are now available in more than 70% of the country's hospitals. Rapid test kits, especially for COVID-19, have seen high demand, with over 5 million units distributed in the last two years. The Ministry of Health is further supporting PoC diagnostics by integrating them into primary healthcare clinics, aiming to reduce diagnostic times across the country.
Scope of the Report
|
By Technology |
PCR NGS Isothermal Amplification DNA Microarray Others |
|
By Application |
Infectious Diseases Oncology Genetic Testing Others |
|
By End-User |
Hospitals Diagnostic Laboratories Research Institutes Home Care Settings |
|
By Product Type |
Instruments Kits & Reagents Software |
|
By Region |
Riyadh Jeddah Dammam Makkah Eastern Province |
Products
Key Target Audience
Hospitals and Healthcare Providers
Diagnostic Laboratories
Medical Device Manufacturers
Pharmaceutical Companies
Investors and Venture Capitalist Firms
Government and Regulatory Bodies (Saudi Food and Drug Authority, Ministry of Health)
Medical Research Institutes
Banks and Financial Institutes
Healthcare Technology Firms
Banks and Financial Institutions
Companies
Major Players in Mentioned in the Report
Roche Diagnostics
Abbott Laboratories
Thermo Fisher Scientific
Qiagen
Agilent Technologies
Danaher Corporation
Illumina, Inc.
Bio-Rad Laboratories
Siemens Healthineers
Al Borg Diagnostics
Almana General Hospital
Cepheid
Beckman Coulter
BioMrieux
Genefluidics, Inc.
Table of Contents
1. KSA Molecular Diagnostics Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate (Molecular Testing Adoption, Diagnostic Lab Growth, Healthcare Expenditure)
1.4. Market Segmentation Overview (Applications, Technology, End-User, Testing Type, Region)
2. KSA Molecular Diagnostics Market Size (In USD Mn)
2.1. Historical Market Size (Test Volume, Market Revenue, Pricing Trends)
2.2. Year-On-Year Growth Analysis (Healthcare Sector Growth, Test Utilization Rates)
2.3. Key Market Developments and Milestones (Healthcare Policy Changes, New Lab Accreditations, Technological Adoption)
3. KSA Molecular Diagnostics Market Analysis
3.1. Growth Drivers
3.1.1. Prevalence of Infectious Diseases (COVID-19, MERS, Tuberculosis)
3.1.2. Technological Advancements (Next-Generation Sequencing, PCR, AI in Diagnostics)
3.1.3. Increasing Focus on Precision Medicine (Genetic Testing, Oncology Diagnostics)
3.1.4. Government Initiatives (Vision 2030, Healthcare Investments, NEOM Healthcare Initiatives)
3.2. Market Challenges
3.2.1. High Costs of Molecular Diagnostic Technologies (PCR, NGS Equipment, Consumables)
3.2.2. Shortage of Skilled Workforce (Laboratory Technicians, Genetic Counselors)
3.2.3. Limited Healthcare Access in Rural Regions (Logistics Challenges, Lab Infrastructure)
3.3. Opportunities
3.3.1. Expansion of Genetic Testing Services (Cancer Genomics, Pre-Natal Screening)
3.3.2. Growth in Point-of-Care Diagnostics (Portable PCR, Rapid Test Kits)
3.3.3. Rise in Public-Private Partnerships (Healthcare Infrastructure, Diagnostics Collaborations)
3.4. Trends
3.4.1. Adoption of Digital Pathology and AI-Based Diagnostics
3.4.2. Growth of Home-Based Testing Solutions (Telemedicine, Home Sample Collection)
3.4.3. Integration of Genomic Data in Electronic Health Records (EHRs)
3.4.4. Increased Government Focus on Diagnostic Self-Sufficiency (Local Production of Diagnostic Equipment)
3.5. Regulatory Framework
3.5.1. Saudi Food and Drug Authority (SFDA) Regulations on Diagnostics
3.5.2. Compliance Requirements for Molecular Diagnostics (International Standards, Accreditation)
3.5.3. Licensing and Certification Processes (Lab Accreditations, Operator Training)
3.6. SWOT Analysis
3.7. Ecosystem and Stakeholder Mapping (Public Hospitals, Private Labs, Equipment Suppliers)
3.8. Porters Five Forces Analysis
3.9. Competitive Landscape
4. KSA Molecular Diagnostics Market Segmentation
4.1. By Application (In Value %)
4.1.1. Infectious Diseases
4.1.2. Oncology
4.1.3. Genetic Testing
4.1.4. Blood Screening
4.1.5. Others
4.2. By Technology (In Value %)
4.2.1. Polymerase Chain Reaction (PCR)
4.2.2. Next-Generation Sequencing (NGS)
4.2.3. In Situ Hybridization
4.2.4. Microarray
4.2.5. Others
4.3. By End-User (In Value %)
4.3.1. Hospitals
4.3.2. Diagnostic Laboratories
4.3.3. Research Institutes
4.3.4. Clinics
4.3.5. Others
4.4. By Testing Type (In Value %)
4.4.1. Centralized Laboratory Testing
4.4.2. Point-of-Care Testing
4.4.3. Home-Based Testing
4.5. By Region (In Value %)
4.5.1. Riyadh
4.5.2. Jeddah
4.5.3. Dammam
4.5.4. Makkah
4.5.5. Eastern Province
5. KSA Molecular Diagnostics Market Competitive Analysis
5.1. Detailed Profiles of Major Competitors
5.1.1. Roche Diagnostics
5.1.2. Abbott Laboratories
5.1.3. Thermo Fisher Scientific
5.1.4. Qiagen
5.1.5. Agilent Technologies
5.1.6. Danaher Corporation
5.1.7. Illumina, Inc.
5.1.8. Bio-Rad Laboratories
5.1.9. Siemens Healthineers
5.1.10. Al Borg Diagnostics
5.1.11. Almana General Hospital
5.1.12. Genefluidics, Inc.
5.1.13. Cepheid
5.1.14. Beckman Coulter
5.1.15. BioMrieux
5.2. Cross Comparison Parameters (No. of Employees, Headquarters, Revenue, Product Portfolio, Market Share, Strategic Initiatives, Partnerships, R&D Investment)
5.3. Market Share Analysis
5.4. Strategic Initiatives (Partnerships, Mergers & Acquisitions, Collaborations)
5.5. Investment Analysis (Private Equity, Venture Capital, Government Grants)
5.6. R&D Investment Trends
6. KSA Molecular Diagnostics Market Regulatory Framework
6.1. SFDA Regulations on Molecular Diagnostics
6.2. Healthcare Accreditation Standards (CBAHI, JCI)
6.3. Certification and Licensing Processes
7. KSA Molecular Diagnostics Future Market Size (In USD Mn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Growth (Advances in Technology, Increasing Healthcare Investments, Growing Prevalence of Chronic Diseases)
8. KSA Molecular Diagnostics Future Market Segmentation
8.1. By Application (In Value %)
8.2. By Technology (In Value %)
8.3. By End-User (In Value %)
8.4. By Testing Type (In Value %)
8.5. By Region (In Value %)
9. KSA Molecular Diagnostics Market Analysts Recommendations
9.1. Market Entry Strategies (Strategic Alliances, Product Launches, Regulatory Approvals)
9.2. White Space Opportunity Analysis
9.3. TAM/SAM/SOM Analysis
9.4. Customer Cohort and Buying Behavior Analysis
Research Methodology
Step 1: Identification of Key Variables
The first step involves mapping the KSA Molecular Diagnostics Market's ecosystem, focusing on identifying key players such as hospitals, diagnostic labs, and healthcare providers. Extensive desk research is conducted using secondary data sources to define crucial market variables, including test adoption rates and technological penetration.
Step 2: Market Analysis and Construction
Historical market data is gathered to analyze penetration levels of molecular diagnostic tests. We examine the revenue generation of diagnostic companies and hospitals while assessing the integration of molecular technologies within the healthcare system.
Step 3: Hypothesis Validation and Expert Consultation
Market hypotheses are formulated and validated through consultations with industry experts and stakeholders. These include diagnostic laboratory professionals and technology suppliers, providing insights that are vital for refining the overall analysis of the market.
Step 4: Research Synthesis and Final Output
The final stage involves synthesizing insights from industry leaders and market participants, ensuring that the data is cross-verified through both top-down and bottom-up approaches. This ensures that the report offers comprehensive and accurate market projections and recommendations.
Frequently Asked Questions
01. How big is the KSA Molecular Diagnostics Market?
The KSA Molecular Diagnostics Market was valued at USD 530 million, driven by increased healthcare investments and the rising demand for advanced diagnostics in hospitals and laboratories.
02. What are the challenges in the KSA Molecular Diagnostics Market?
Key challenges in the KSA Molecular Diagnostics Market include the high cost of molecular diagnostic equipment and consumables, as well as the shortage of trained professionals capable of operating these advanced technologies.
03. Who are the major players in the KSA Molecular Diagnostics Market?
Key players in the KSA Molecular Diagnostics Market include Roche Diagnostics, Abbott Laboratories, Thermo Fisher Scientific, and local players such as Al Borg Diagnostics, all of whom have significant influence over the market through strategic partnerships and product offerings.
04. What are the growth drivers of the KSA Molecular Diagnostics Market?
The KSA Molecular Diagnostics market is propelled by the increasing prevalence of infectious diseases, government investments in healthcare infrastructure under Vision 2030, and the adoption of advanced molecular technologies like PCR and NGS.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















