
KSA Orthopedic Devices Market Outlook to 2030
Region:Middle East
Author(s):Yogita Sahu
Product Code:KROD6787
November 2024
96
About the Report
KSA Orthopedic Devices Market Overview
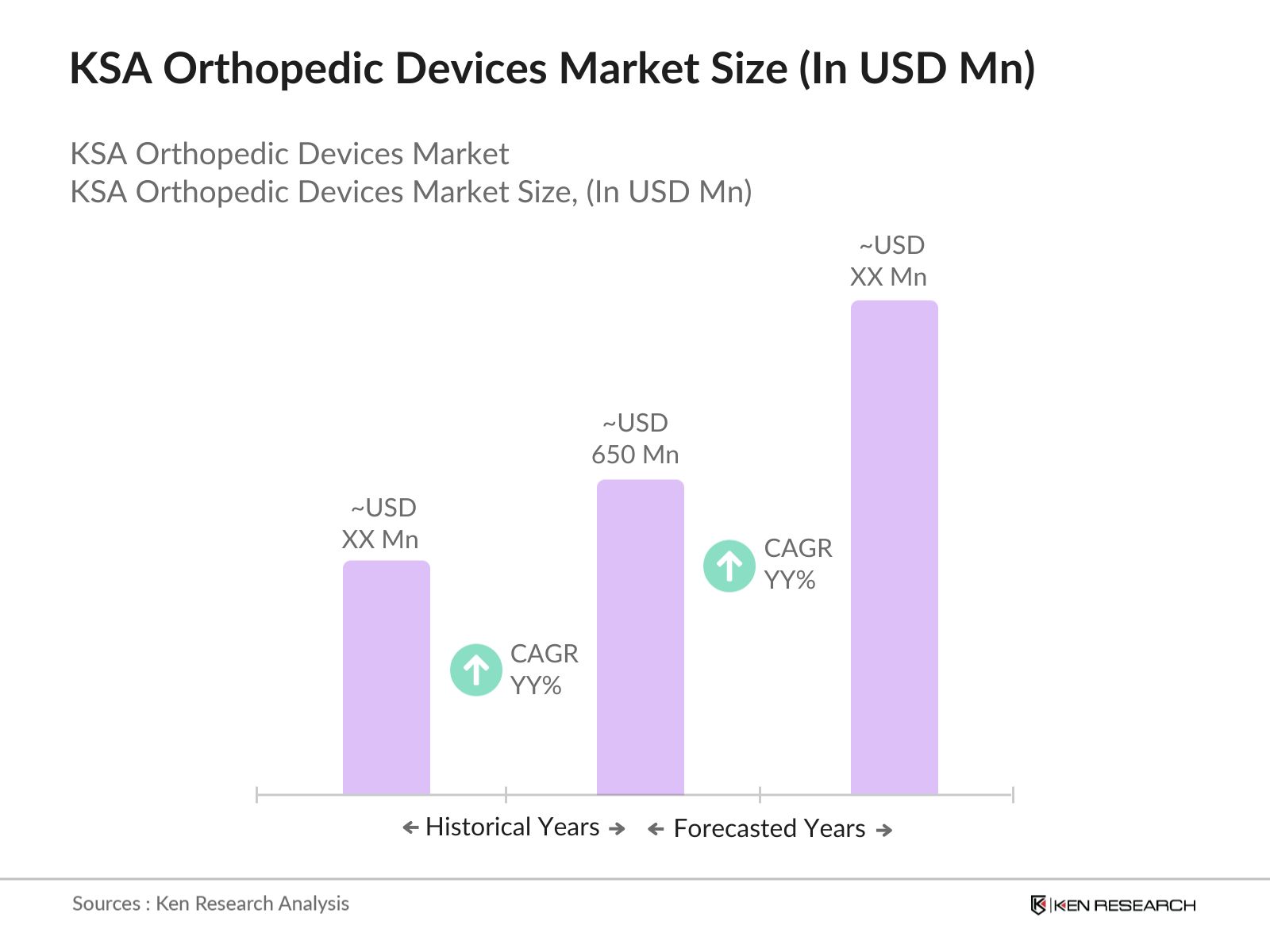
- The KSA orthopedic devices market is valued at USD 650 million, driven by an increasing aging population and rising cases of orthopedic disorders. The growing need for joint replacements, coupled with the rise of medical tourism in Saudi Arabia, has accelerated the markets growth. Government investments in healthcare infrastructure, particularly in orthopedics, have further boosted demand for advanced medical devices in hospitals and clinics.
- In terms of regional dominance, Riyadh and Jeddah are leading hubs in the market. Riyadh's dominance is attributed to the high concentration of advanced healthcare facilities, major hospitals, and healthcare professionals. Jeddah is also a key player due to its growing medical tourism sector, which attracts patients from neighboring countries seeking high-quality orthopedic care at lower costs.
- Under the Vision 2030 initiative, KSA's healthcare sector received SAR 180 billion in 2024, with a portion allocated for upgrading orthopedic care and increasing access to advanced surgical technologies. The government also supports public-private partnerships, attracting international orthopedic device manufacturers to establish local production units and joint ventures.
KSA Orthopedic Devices Market Segmentation
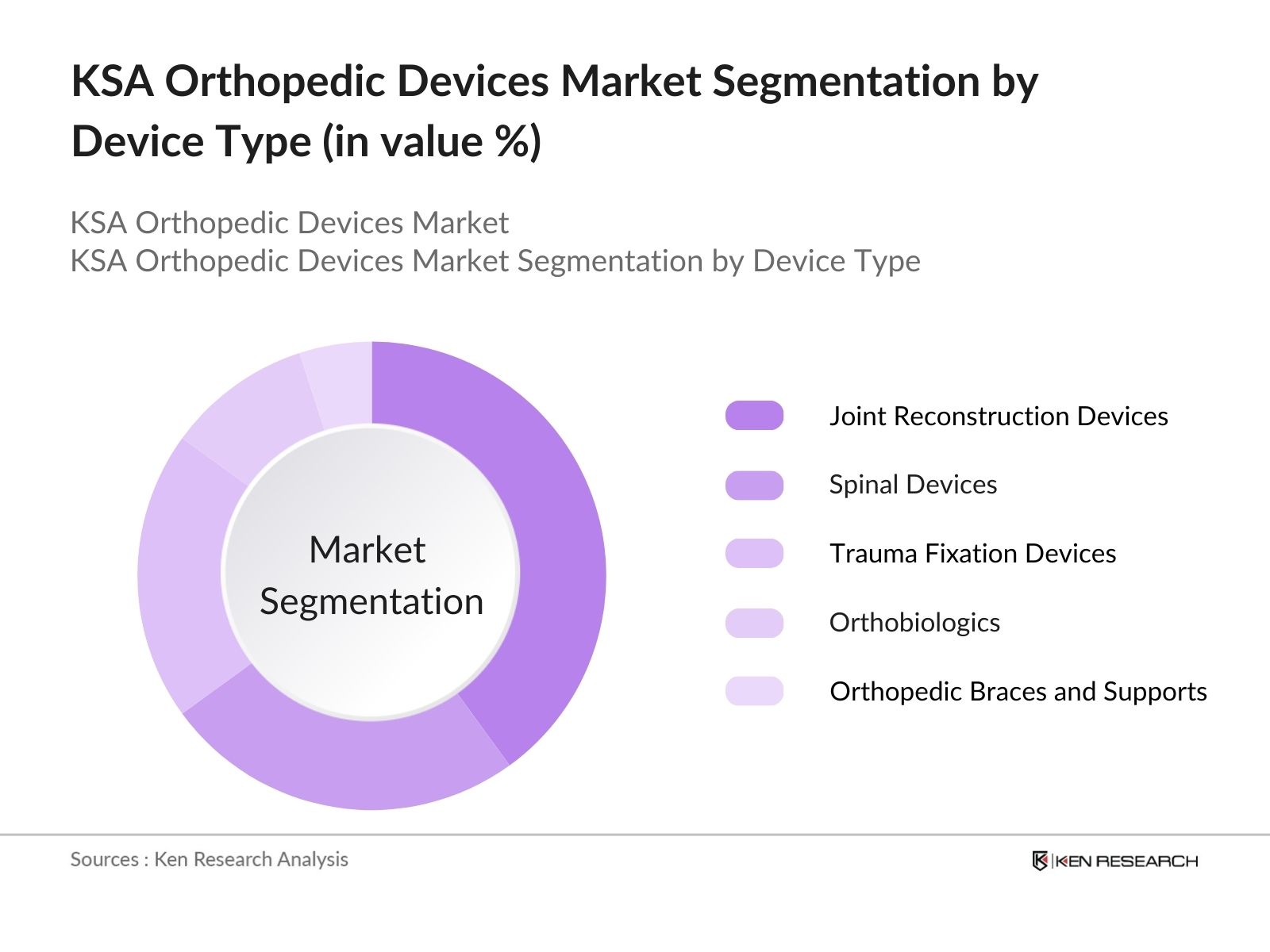
By Device Type: The market is segmented by device type into joint reconstruction devices, spinal devices, trauma fixation devices, orthobiologics, and orthopedic braces and supports. Recently, joint reconstruction devices have emerged as the dominant sub-segment, particularly due to the rising cases of knee and hip replacements in the elderly population. With advancements in prosthetic technologies, the demand for these devices continues to grow, especially as more patients seek minimally invasive surgeries for joint reconstruction.
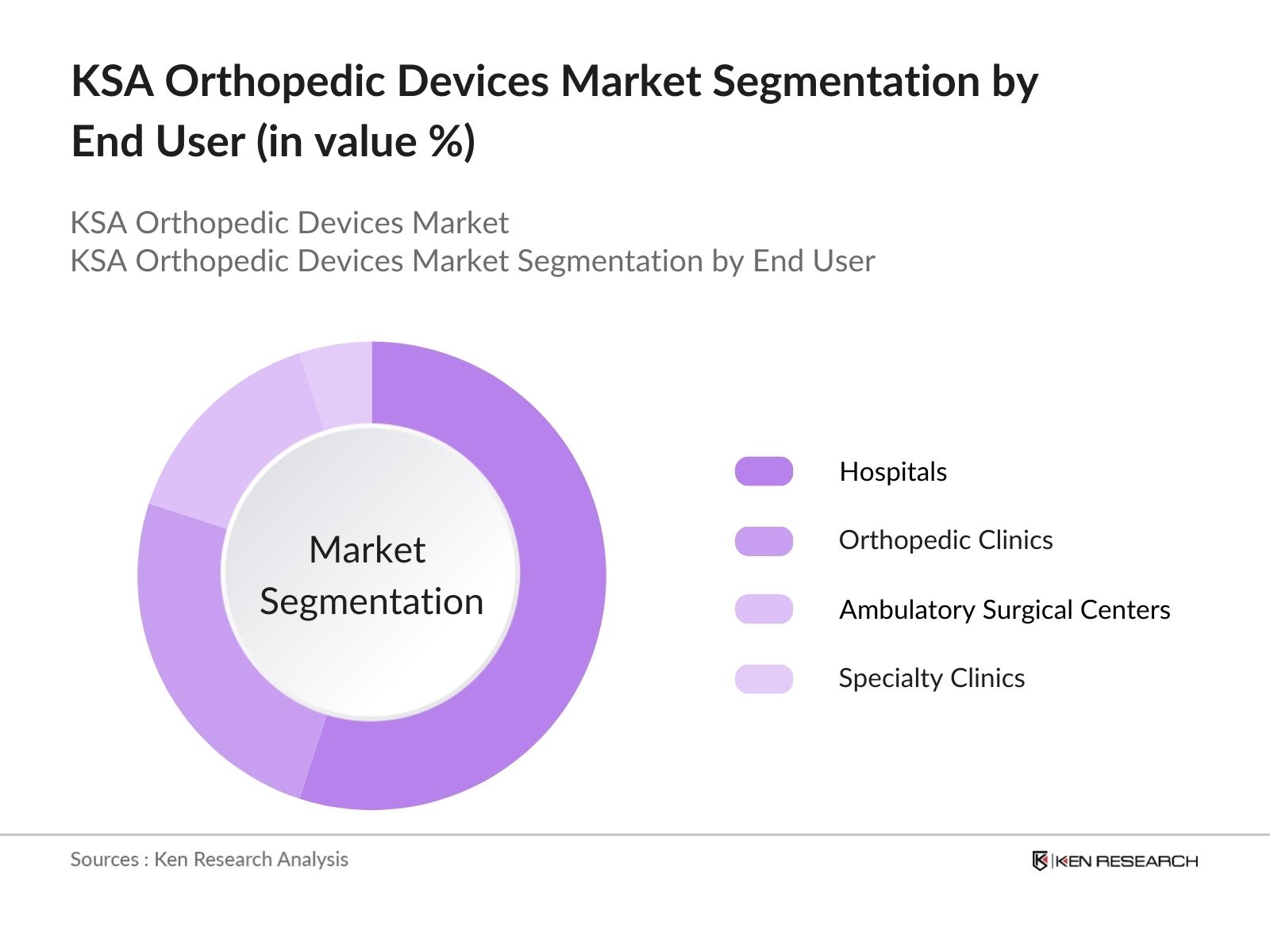
By End User: The market is also segmented by end user into hospitals, orthopedic clinics, ambulatory surgical centers, and specialty clinics. Hospitals are currently leading this segment due to their large patient inflow and superior medical facilities. As government funding increases and more hospitals are equipped with state-of-the-art technology, they continue to serve as the primary setting for complex orthopedic procedures such as joint replacements and spinal surgeries.
KSA Orthopedic Devices Market Competitive Landscape
The market is dominated by a mix of global and local players. The presence of established international companies and local manufacturers has led to intense competition. Notably, foreign companies dominate the high-end device segment, while local firms are gaining traction in cost-sensitive segments such as orthopedic braces and supports.
|
Company Name |
Established |
Headquarters |
No. of Employees |
R&D Expenditure |
Product Range |
Revenue (SAR Bn) |
Partnerships |
Mergers/Acquisitions |
|
Zimmer Biomet |
1927 |
USA |
||||||
|
Stryker Corporation |
1941 |
USA |
||||||
|
Medtronic |
1949 |
Ireland |
||||||
|
DePuy Synthes |
1895 |
USA |
||||||
|
Arthrex, Inc. |
1981 |
USA |
KSA Orthopedic Devices Market Analysis
Market Growth Drivers
- Increasing Prevalence of Musculoskeletal Disorders: In 2024, the Kingdom of Saudi Arabia (KSA) reports over 4.8 million patients suffering from osteoarthritis and other musculoskeletal conditions, making these disorders a significant public health concern. With the growing aging populationaround 2.5 million people above 65 yearsdemand for orthopedic devices like joint reconstruction implants is rising sharply, particularly for knee and hip replacements. Hospitals in KSA report a 12% annual increase in orthopedic surgeries, further fueling the demand for devices.
- Government Healthcare Expansion Initiatives: The KSA government allocated SAR 180 billion to healthcare in 2024, with SAR 20 billion specifically aimed at medical infrastructure upgrades and device procurement, including orthopedic devices. Under Vision 2030, the government is promoting investment in the medical sector, leading to increased adoption of orthopedic devices across public hospitals. By 2024, the government also initiated a national registry for orthopedic implants, ensuring device tracking and increasing transparency.
- Increasing Road Traffic Accidents: Saudi Arabia experiences over 600,000 road traffic accidents annually, resulting in around 39,000 severe injuries requiring orthopedic treatments such as bone fixation devices and spinal implants. In 2024, over 18,000 surgeries for fracture fixation were conducted in major hospitals, driving the demand for trauma-related orthopedic devices.
Market Challenges
- Regulatory Compliance and Delays: As of 2024, Saudi Arabia has strengthened its regulatory framework for medical devices, including the orthopedic sector, requiring more stringent approvals for new products. However, the process can take 6-12 months for device approvals, leading to delays in product launches. This extended regulatory process impacts market growth and availability of innovative devices.
- Lack of Skilled Orthopedic Surgeons: While the number of orthopedic surgeries is increasing, KSA faces a shortage of highly trained orthopedic surgeons. In 2024, there are approximately 1,200 certified orthopedic surgeons in the country, but demand exceeds supply, particularly in public hospitals. This gap leads to longer waiting times for surgeries, reducing the effectiveness of orthopedic treatments and delaying the adoption of advanced devices.
KSA Orthopedic Devices Market Future Outlook
Over the next five years, the KSA orthopedic devices industry is expected to exhibit growth due to increasing healthcare spending, the rise in chronic conditions like arthritis, and the high demand for advanced medical procedures. Government efforts under Vision 2030 to enhance healthcare services and encourage medical tourism will continue to drive investments in orthopedic care.
Future Market Opportunities
- Increase in Local Manufacturing of Orthopedic Devices: By 2029, it is anticipated that local manufacturing of orthopedic devices will meet 30% of the domestic demand. Government initiatives and private sector partnerships are expected to reduce import dependency, enhancing the affordability of devices and expanding access to rural regions.
- Adoption of 3D-Printed Orthopedic Solutions: The adoption of 3D printing technology in orthopedic implants is forecasted to accelerate, with estimates indicating that by 2029, over 20% of joint replacement surgeries in KSA will use 3D-printed custom implants. This trend will be driven by advancements in material science and increasing collaboration between hospitals and tech firms.
Scope of the Report
|
By Device Type |
Joint Reconstruction Devices Spinal Devices Trauma Fixation Devices Ortho biologics Orthopedic Braces and Supports |
|
By End User |
Hospitals Orthopedic Clinics Ambulatory Surgical Centers Specialty Clinics |
|
By Application |
Knee Replacement Hip Replacement Spine Surgery Sports Medicine Trauma |
|
By Technology |
Conventional Orthopedic Devices Robotic-Assisted Devices Computer-Assisted Surgery Devices 3D Printed Orthopedic Devices |
|
By Region |
North West East South |
Products
Key Target Audience Organizations and Entities Who Can Benefit by Subscribing This Report:
Orthopedic Device Manufacturers
Hospitals and Healthcare Providers
Government and Regulatory Bodies (e.g., Saudi Food & Drug Authority)
Banks and Financial Institution
Investor and Venture Capitalist Firms
Insurance Providers
Private Equity Firms
Companies
Players Mentioned in the Report:
Zimmer Biomet
Stryker Corporation
Medtronic
DePuy Synthes
Arthrex, Inc.
Smith & Nephew
NuVasive, Inc.
DJO Global
B. Braun Melsungen AG
Aesculap Implant Systems
Table of Contents
1. KSA Orthopedic Devices Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate
1.4. Market Segmentation Overview
2. KSA Orthopedic Devices Market Size (in USD Mn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis
2.3. Key Market Developments and Milestones
3. KSA Orthopedic Devices Market Analysis
3.1. Growth Drivers (Orthopedic Procedures, Aging Population, Healthcare Infrastructure, Medical Tourism)
3.2. Market Challenges (High Cost of Advanced Devices, Reimbursement Policies, Regulatory Barriers, Low Healthcare Penetration in Rural Areas)
3.3. Opportunities (3D Printing, Robotic-Assisted Surgeries, Public-Private Partnerships, Healthcare Reforms)
3.4. Trends (Increased Adoption of Minimally Invasive Surgery, Usage of Smart Orthopedic Implants, Outpatient Surgeries Growth, Telehealth Integration)
3.5. Government Regulations (SFDA Medical Device Regulation, Public Healthcare Spending Initiatives, Local Manufacturing Incentives, Import Tariffs)
3.6. SWOT Analysis (Strengths, Weaknesses, Opportunities, Threats)
3.7. Stakeholder Ecosystem (Hospitals, Clinics, Distributors, Manufacturers, Insurance Providers)
3.8. Porters Five Forces (Bargaining Power of Suppliers, Bargaining Power of Buyers, Threat of New Entrants, Threat of Substitutes, Industry Rivalry)
3.9. Competition Ecosystem
4. KSA Orthopedic Devices Market Segmentation (In Value %)
4.1. By Device Type
4.1.1. Joint Reconstruction Devices
4.1.2. Spinal Devices
4.1.3. Trauma Fixation Devices
4.1.4. Orthobiologics
4.1.5. Orthopedic Braces and Supports
4.2. By End User
4.2.1. Hospitals
4.2.2. Orthopedic Clinics
4.2.3. Ambulatory Surgical Centers
4.2.4. Specialty Clinics
4.3. By Application
4.3.1. Knee Replacement
4.3.2. Hip Replacement
4.3.3. Spine Surgery
4.3.4. Sports Medicine
4.3.5. Trauma
4.4. By Technology
4.4.1. Conventional Orthopedic Devices
4.4.2. Robotic-Assisted Devices
4.4.3. Computer-Assisted Surgery Devices
4.4.4. 3D Printed Orthopedic Devices
4.5. By Region
4.5.1. Central Province
4.5.2. Western Province
4.5.3. Eastern Province
4.5.4. Northern Province
5. KSA Orthopedic Devices Market Competitive Analysis
5.1. Detailed Profiles of Major Companies
5.1.1. Zimmer Biomet
5.1.2. DePuy Synthes
5.1.3. Stryker Corporation
5.1.4. Smith & Nephew
5.1.5. Medtronic
5.1.6. NuVasive, Inc.
5.1.7. DJO Global
5.1.8. B. Braun Melsungen AG
5.1.9. Aesculap Implant Systems
5.1.10. Arthrex, Inc.
5.1.11. Globus Medical
5.1.12. Wright Medical Group N.V.
5.1.13. ConMed Corporation
5.1.14. Orthofix International
5.1.15. Exactech, Inc.
5.2. Cross Comparison Parameters (No. of Employees, Headquarters, Revenue, Market Share, Key Partnerships, Research and Development Expenditure, Product Launches, Innovation Strategy)
5.3. Market Share Analysis
5.4. Strategic Initiatives (Mergers & Acquisitions, Partnerships, Collaborations, Licensing)
5.5. Investment Analysis
5.6. Venture Capital Funding
5.7. Government Grants
5.8. Private Equity Investments
6. KSA Orthopedic Devices Market Regulatory Framework
6.1. Compliance with SFDA Standards
6.2. Import Regulations and Local Manufacturing Policies
6.3. Certification Processes
6.4. Medical Device Registration Requirements
7. KSA Orthopedic Devices Future Market Size (in USD Mn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth
8. KSA Orthopedic Devices Future Market Segmentation (In Value %)
8.1. By Device Type
8.2. By End User
8.3. By Application
8.4. By Technology
8.5. By Region
9. KSA Orthopedic Devices Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Customer Cohort Analysis
9.3. Marketing Initiatives
9.4. White Space Opportunity Analysis
Research Methodology
Step 1: Identification of Key Variables
The first step involves mapping the entire orthopedic devices ecosystem in Saudi Arabia. This includes identifying key stakeholders such as manufacturers, suppliers, hospitals, clinics, and regulatory bodies. Extensive desk research using proprietary databases and government reports was undertaken to establish critical market variables, including device types and usage trends.
Step 2: Market Analysis and Construction
Historical data from the last five years was analyzed, focusing on market penetration, hospital usage rates, and revenue generation. An evaluation of healthcare infrastructure and the increasing adoption of robotic-assisted surgeries was conducted to ensure the accuracy of future market projections.
Step 3: Hypothesis Validation and Expert Consultation
Hypotheses related to the growth of the orthopedic devices market were validated through structured interviews with experts from leading orthopedic device companies and healthcare professionals in Saudi Arabia. These consultations provided essential insights into market trends and operational challenges.
Step 4: Research Synthesis and Final Output
The final phase involved a synthesis of research findings, complemented by interactions with healthcare providers and orthopedic specialists. This helped to validate key data points and refine the final market estimates, ensuring an accurate and comprehensive market analysis.
Frequently Asked Questions
01. How big is the KSA Orthopedic Devices Market?
The KSA orthopedic devices market is valued at USD 650 million, driven by the increasing demand for joint reconstruction devices and a growing aging population in the country.
02. What are the challenges in the KSA Orthopedic Devices Market?
Challenges in the KSA orthopedic devices market include the high cost of advanced devices, complex regulatory procedures, and the limited availability of skilled professionals for robotic-assisted surgeries.
03. Who are the major players in the KSA Orthopedic Devices Market?
Major players in the KSA orthopedic devices market include Zimmer Biomet, Stryker Corporation, Medtronic, DePuy Synthes, and Arthrex, Inc., dominating due to their advanced product offerings and extensive distribution networks.
04. What are the growth drivers of the KSA Orthopedic Devices Market?
Key growth drivers in the KSA orthopedic devices market include government investments in healthcare infrastructure, the rising incidence of joint-related disorders, and the growing demand for minimally invasive surgeries.
05. What are the future trends in the KSA Orthopedic Devices Market?
The future of the KSA orthopedic devices market will be shaped by technological advancements like 3D printing and robotic surgeries, which offer more precise and customized treatments.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















