Region:Middle East
Author(s):Geetanshi
Product Code:KRAD7330
Pages:89
Published On:December 2025
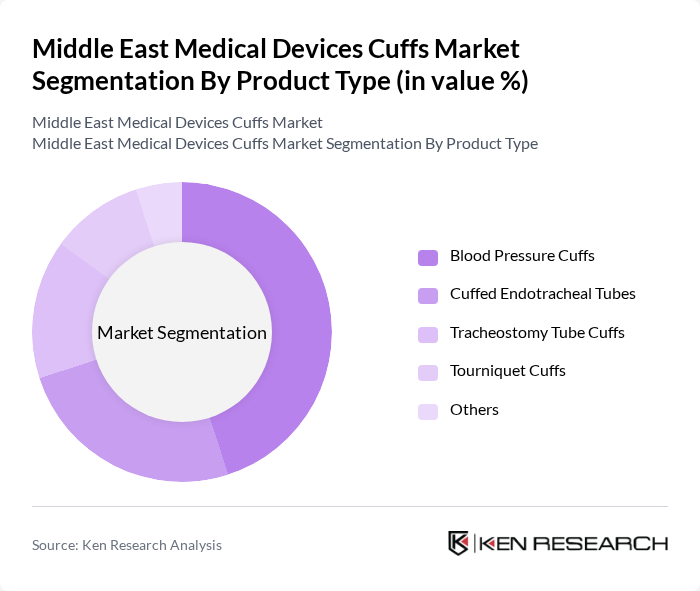
By Product Type:The product type segmentation includes various categories such as Blood Pressure Cuffs, Cuffed Endotracheal Tubes, Tracheostomy Tube Cuffs, Tourniquet Cuffs, and Others. Among these, Blood Pressure Cuffs are the most widely used due to their essential role in non-invasive blood pressure monitoring, which is critical in both hospital and home healthcare settings and is supported by the growing adoption of automated and digital sphygmomanometers in the region. The increasing prevalence of hypertension and cardiovascular diseases, together with expanded screening programs and remote patient monitoring initiatives, drives the demand for these cuffs, making them a dominant segment in the market.
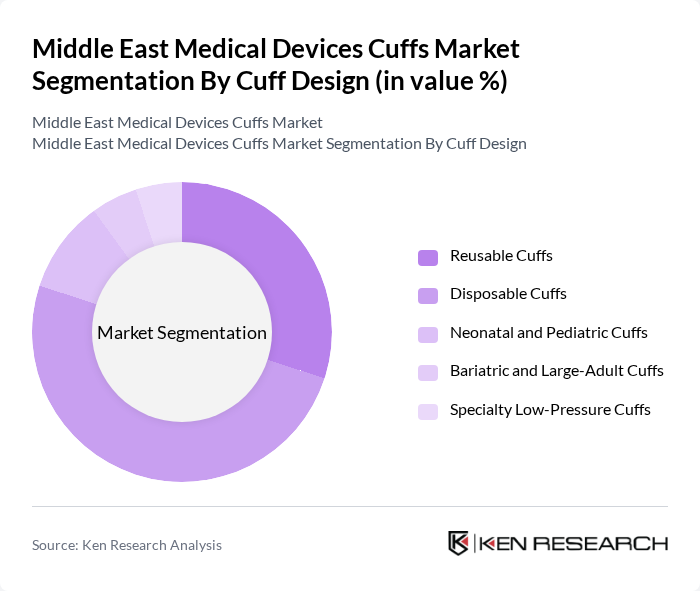
By Cuff Design:The cuff design segmentation includes Reusable Cuffs, Disposable Cuffs, Neonatal and Pediatric Cuffs, Bariatric and Large-Adult Cuffs, and Specialty Low-Pressure Cuffs. Disposable Cuffs are gaining traction due to their convenience and reduced risk of cross-contamination, especially in hospital and ambulatory surgery center settings, where infection prevention and control protocols have been strengthened after the pandemic. The growing emphasis on infection control, patient safety, and single?patient?use devices, combined with procurement policies that prioritize hygienic and traceable supplies, is driving the demand for disposable options, making them a leading segment in the market.
The Middle East Medical Devices Cuffs Market is characterized by a dynamic mix of regional and international players. Leading participants such as Omron Healthcare, Inc., Koninklijke Philips N.V. (Philips Healthcare), GE HealthCare Technologies Inc., Medtronic plc, A&D Company, Limited (A&D Medical), Hillrom (Welch Allyn brand, a Baxter company), SunTech Medical, Inc., Nihon Kohden Corporation, Spacelabs Healthcare (OSI Systems, Inc.), Schiller AG, Shenzhen Mindray Bio?Medical Electronics Co., Ltd., ICU Medical, Inc. (including former Welch Allyn BP components), Smiths Medical (ICU Medical) – Cuff and Airway Management Products, Drägerwerk AG & Co. KGaA, Becton, Dickinson and Company (BD) – Vascular Access and Pressure Cuffs contribute to innovation, geographic expansion, and service delivery in this space, particularly through non?invasive blood pressure systems, anesthesia and ventilation circuits, and critical care monitoring platforms that integrate cuff technologies.
The future of the Middle East medical devices cuffs market appears promising, driven by ongoing innovations and a shift towards patient-centric healthcare solutions. As telemedicine continues to expand, the integration of remote monitoring technologies will likely enhance the demand for advanced cuffs. Additionally, the increasing focus on preventive healthcare will encourage investments in devices that facilitate early diagnosis and management of chronic diseases, further propelling market growth in the coming years.
| Segment | Sub-Segments |
|---|---|
| By Product Type | Blood Pressure Cuffs Cuffed Endotracheal Tubes Tracheostomy Tube Cuffs Tourniquet Cuffs Others |
| By Cuff Design | Reusable Cuffs Disposable Cuffs Neonatal and Pediatric Cuffs Bariatric and Large-Adult Cuffs Specialty Low?Pressure Cuffs |
| By End-User | Hospitals Clinics and Physician Offices Ambulatory Surgery Centers Home Healthcare Settings Others |
| By Distribution Channel | Direct Tenders and Institutional Sales Medical Device Distributors Online B2B / E?Procurement Portals Retail and Hospital Pharmacies Others |
| By Clinical Application | Non?Invasive Blood Pressure Monitoring Anesthesia and Airway Management Critical Care and ICU Monitoring Emergency and Transport Monitoring Others |
| By Technology | Manual / Aneroid Cuff Systems Automated Oscillometric Systems Integrated Multi?Parameter Monitor Cuffs Bluetooth / Connected and Wearable Cuffs Others |
| By Country | Saudi Arabia United Arab Emirates Qatar Kuwait Oman Rest of Middle East |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Hospital Procurement Departments | 100 | Procurement Managers, Supply Chain Coordinators |
| Healthcare Professionals | 120 | Doctors, Nurses, Medical Technicians |
| Medical Device Distributors | 80 | Sales Managers, Product Specialists |
| Patient User Experience | 70 | Patients, Caregivers, Health Advocates |
| Regulatory Bodies | 50 | Regulatory Affairs Specialists, Compliance Officers |
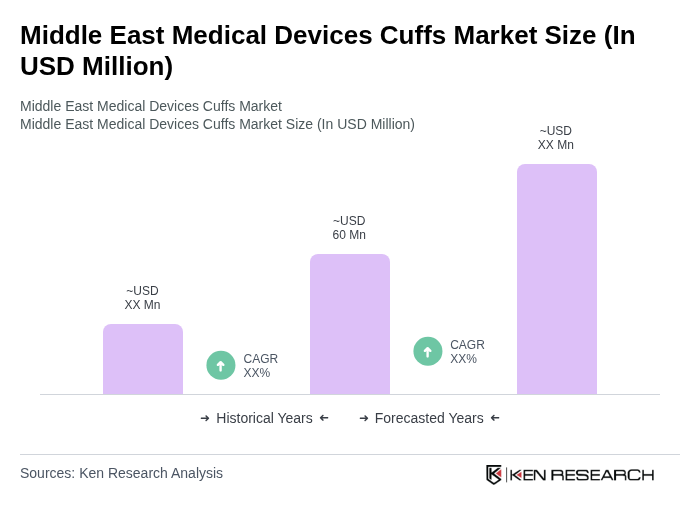
The Middle East Medical Devices Cuffs Market is valued at approximately USD 60 million, driven by the rising prevalence of chronic diseases and increasing healthcare expenditures in the region, particularly in Gulf Cooperation Council (GCC) countries.