Region:Middle East
Author(s):Shubham
Product Code:KRAC4315
Pages:80
Published On:October 2025
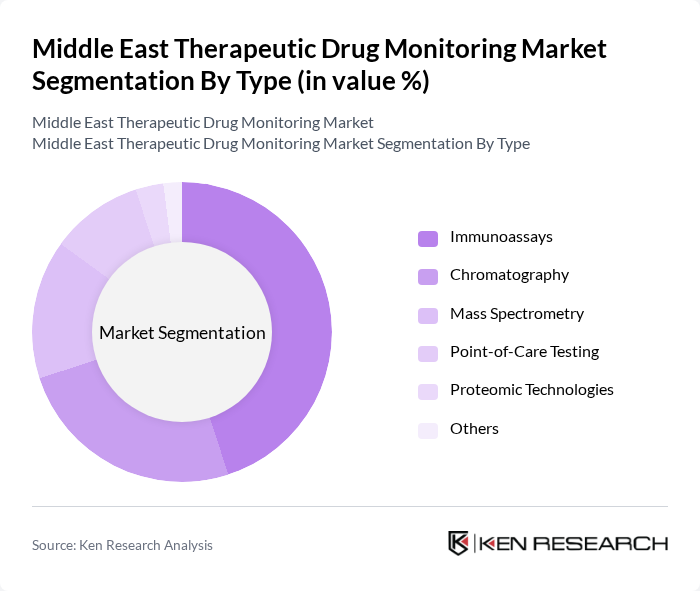
By Type:The market is segmented into Immunoassays, Chromatography, Mass Spectrometry, Point-of-Care Testing, Proteomic Technologies, and Others. Immunoassays remain the leading sub-segment due to their widespread use in clinical laboratories for routine drug level monitoring. Their ease of use, rapid turnaround time, and cost-effectiveness make them the preferred choice for healthcare providers. Chromatography and Mass Spectrometry are increasingly adopted for their superior accuracy and sensitivity, especially in complex cases and for drugs with narrow therapeutic ranges. Point-of-care testing is gaining traction in outpatient and emergency settings for immediate drug level assessment. Proteomic technologies, while still emerging, offer potential for personalized medicine applications and biomarker discovery.
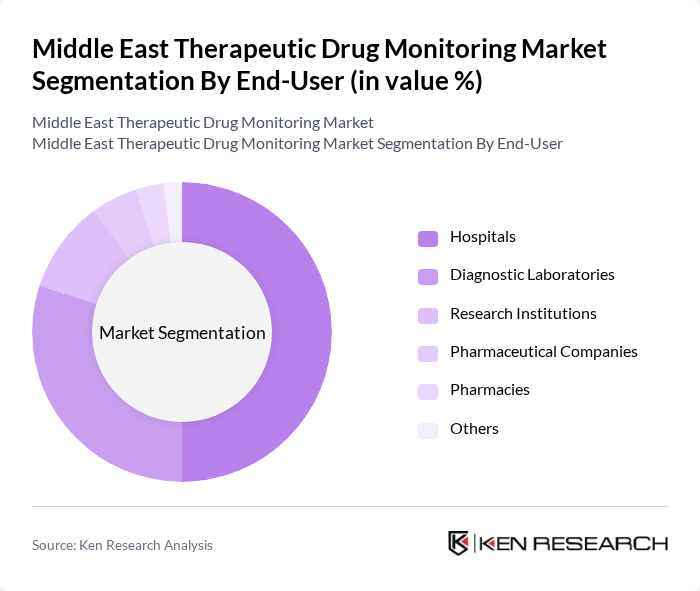
By End-User:The end-user segmentation includes Hospitals, Diagnostic Laboratories, Research Institutions, Pharmaceutical Companies, Pharmacies, and Others. Hospitals are the dominant end-user segment, driven by the increasing number of patients requiring therapeutic drug monitoring for chronic conditions and the integration of advanced diagnostic platforms. Diagnostic laboratories are essential for providing specialized testing services and supporting hospital workflows. Research institutions contribute to innovation and clinical validation of new monitoring techniques. Pharmaceutical companies utilize therapeutic drug monitoring for clinical trials and drug development, while pharmacies and other providers play a supporting role in patient management and medication safety.
The Middle East Therapeutic Drug Monitoring Market is characterized by a dynamic mix of regional and international players. Leading participants such as Abbott Laboratories, Roche Diagnostics, Thermo Fisher Scientific, Siemens Healthineers, Bio-Rad Laboratories, Quest Diagnostics, LabCorp, PerkinElmer, Agilent Technologies, Becton, Dickinson and Company (BD), Beckman Coulter, Inc., Chromsystems Instruments & Chemicals GmbH, Randox Laboratories Ltd., bioMérieux SA, Alere Inc., Drägerwerk AG & Co. KGaA, Kinesis Ltd., Mayo Clinic Laboratories contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Middle East therapeutic drug monitoring market appears promising, driven by technological advancements and increasing healthcare investments. As healthcare systems evolve, the integration of artificial intelligence and telemedicine is expected to enhance TDM practices, making them more accessible and efficient. Furthermore, the growing emphasis on patient-centric healthcare solutions will likely lead to improved monitoring protocols, ensuring better management of chronic diseases and personalized treatment plans for patients across the region.
| Segment | Sub-Segments |
|---|---|
| By Type | Immunoassays Chromatography Mass Spectrometry Point-of-Care Testing Proteomic Technologies Others |
| By End-User | Hospitals Diagnostic Laboratories Research Institutions Pharmaceutical Companies Pharmacies Others |
| By Application | Oncology Psychiatry Infectious Diseases Cardiovascular Diseases Transplant Medicine Others |
| By Distribution Channel | Direct Sales Online Sales Distributors Retail Pharmacies Others |
| By Region | GCC Countries Levant Region North Africa Others |
| By Pricing Model | Subscription-Based Pay-Per-Use Bundled Services Others |
| By Technology | Automated Systems Manual Systems Hybrid Systems Companion Diagnostics Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Clinical Laboratories | 60 | Laboratory Directors, Quality Assurance Managers |
| Healthcare Providers | 50 | Physicians, Pharmacists, Nurse Practitioners |
| Pharmaceutical Companies | 40 | Product Managers, Clinical Research Associates |
| Regulatory Bodies | 40 | Policy Makers, Regulatory Affairs Specialists |
| Patient Advocacy Groups | 40 | Advocacy Leaders, Patient Representatives |
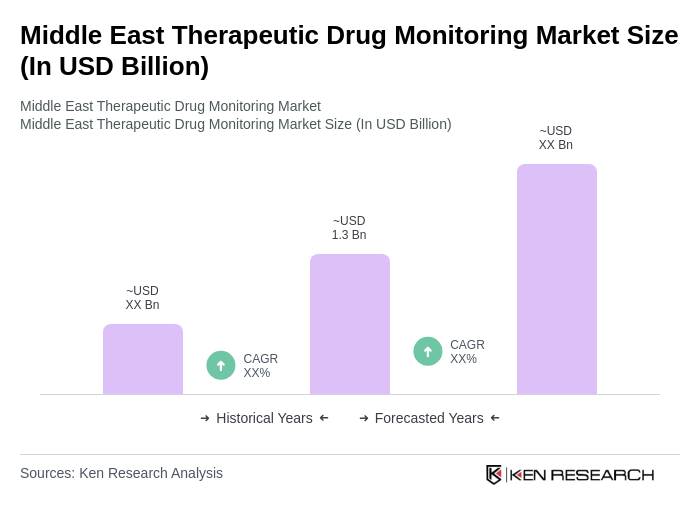
The Middle East Therapeutic Drug Monitoring Market is valued at approximately USD 1.3 billion, reflecting significant growth driven by the rising prevalence of chronic diseases and advancements in diagnostic technologies.