Region:Middle East
Author(s):Dev
Product Code:KRAB7825
Pages:81
Published On:October 2025
By Type:
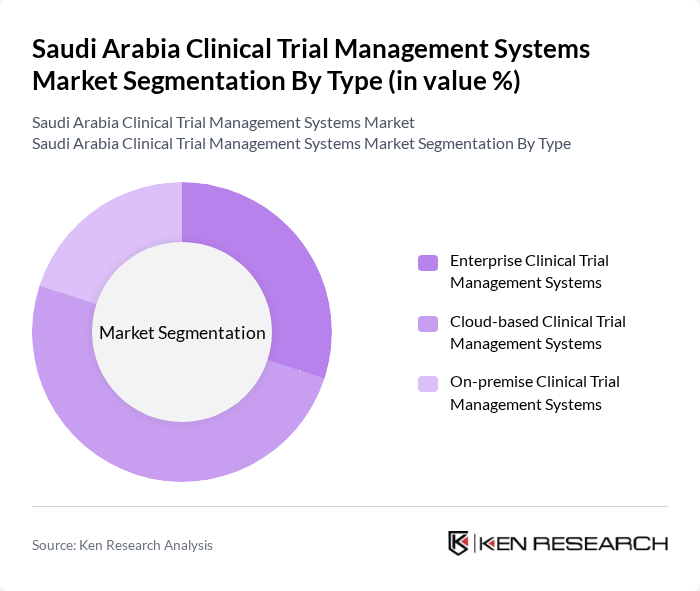
The market is segmented into three main types: Enterprise Clinical Trial Management Systems, Cloud-based Clinical Trial Management Systems, and On-premise Clinical Trial Management Systems. Among these, Cloud-based Clinical Trial Management Systems are leading the market due to their flexibility, scalability, and cost-effectiveness. The increasing adoption of cloud technology in healthcare allows for real-time data access and collaboration among stakeholders, making it a preferred choice for many organizations.
By End-User:
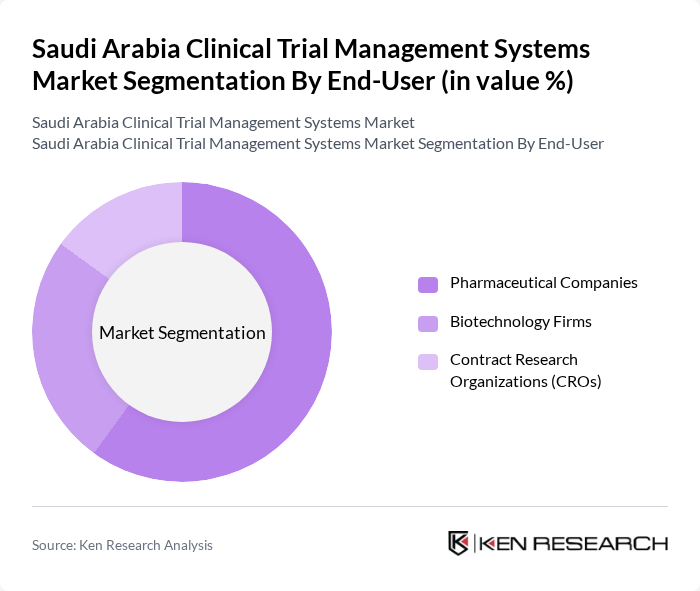
The end-user segmentation includes Pharmaceutical Companies, Biotechnology Firms, and Contract Research Organizations (CROs). Pharmaceutical Companies dominate the market as they are the primary sponsors of clinical trials, driving the demand for effective management systems. The need for efficient data handling and regulatory compliance in drug development processes further solidifies their leading position in the market.
The Saudi Arabia Clinical Trial Management Systems Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medidata Solutions, Inc., Oracle Corporation, Veeva Systems Inc., Parexel International Corporation, Covance Inc., BioClinica, Inc., Medpace, Inc., CRF Health, ERT, Inc., PPD, Inc., Syneos Health, Inc., Clinipace Worldwide, WCG Clinical, ICON plc, KCR contribute to innovation, geographic expansion, and service delivery in this space.
The future of the clinical trial management systems market in Saudi Arabia appears promising, driven by technological advancements and a growing emphasis on patient-centric approaches. As the healthcare sector continues to evolve, the integration of artificial intelligence and machine learning into CTMS is expected to enhance data management and analysis. Additionally, the shift towards decentralized clinical trials will facilitate broader patient participation, ultimately improving trial outcomes and efficiency in the region.
| Segment | Sub-Segments |
|---|---|
| By Type | Enterprise Clinical Trial Management Systems Cloud-based Clinical Trial Management Systems On-premise Clinical Trial Management Systems |
| By End-User | Pharmaceutical Companies Biotechnology Firms Contract Research Organizations (CROs) |
| By Application | Site Management Patient Recruitment Data Management |
| By Deployment Mode | Cloud Deployment On-Premise Deployment |
| By Region | Central Region Western Region Eastern Region |
| By Regulatory Compliance | FDA Compliance EMA Compliance |
| By Others | Niche Solutions Customizable Platforms |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Pharmaceutical Companies | 100 | Clinical Research Managers, Regulatory Affairs Specialists |
| Biotech Firms | 80 | Product Development Managers, Clinical Operations Directors |
| Healthcare Institutions | 90 | Clinical Trial Coordinators, Research Ethics Committee Members |
| Contract Research Organizations (CROs) | 70 | Project Managers, Business Development Executives |
| Regulatory Bodies | 50 | Policy Makers, Compliance Officers |
The Saudi Arabia Clinical Trial Management Systems Market is valued at approximately USD 150 million, reflecting significant growth driven by an increase in clinical trials, investments in research and development, and advancements in technology.