Region:Middle East
Author(s):Shubham
Product Code:KRAD5500
Pages:94
Published On:December 2025
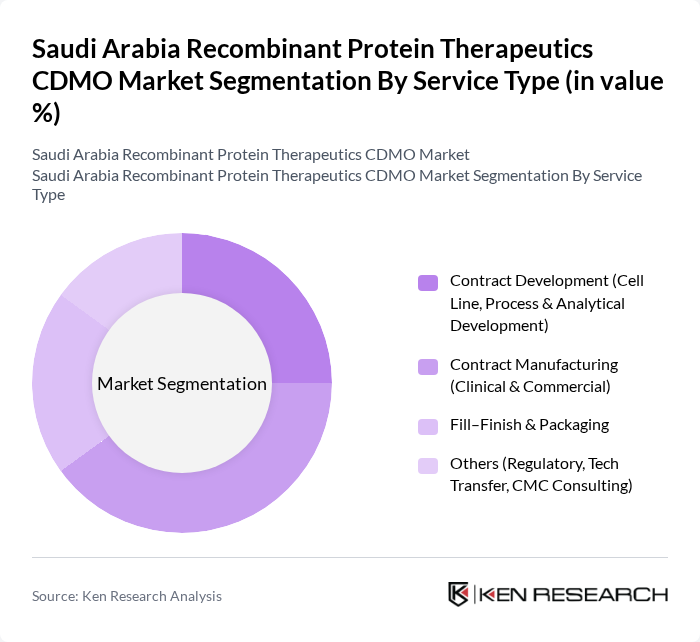
By Service Type:The service type segmentation includes various categories such as Contract Development, Contract Manufacturing, Fill-Finish & Packaging, and Others. Among these, Contract Manufacturing is the leading sub-segment due to the increasing outsourcing of production processes by pharmaceutical companies. This trend is driven by the need for cost efficiency and access to advanced manufacturing technologies including single-use bioreactors and continuous processing. The demand for Fill-Finish services is also growing as companies focus on ensuring the quality and safety of their products before market release.
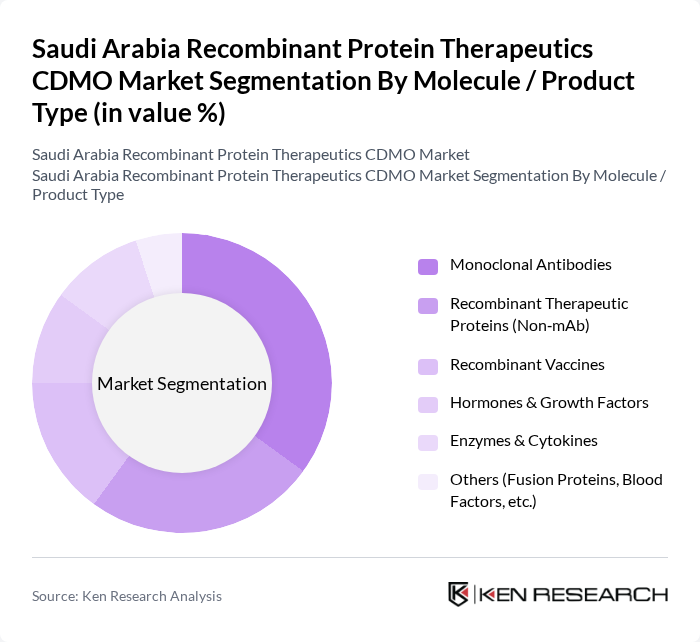
By Molecule / Product Type:The molecule/product type segmentation encompasses Monoclonal Antibodies, Recombinant Therapeutic Proteins (Non-mAb), Recombinant Vaccines, Hormones & Growth Factors, Enzymes & Cytokines, and Others. Monoclonal Antibodies dominate this segment due to their widespread application in treating various diseases, including cancer and autoimmune disorders. The increasing investment in research and development of monoclonal antibody therapies is driving their market share, while the demand for Recombinant Vaccines is also on the rise, particularly in response to global health challenges.
The Saudi Arabia Recombinant Protein Therapeutics CDMO Market is characterized by a dynamic mix of regional and international players. Leading participants such as Lifera (Saudi Arabian Biopharmaceutical Company), Saudi Bio, Kingdom of Saudi Arabia, Tabuk Pharmaceuticals Manufacturing Company, Riyadh Pharma (Saudi Pharmaceutical Industries & Medical Appliances Corporation), Saudi Pharmaceutical Industries and Medical Appliances Corporation (SPIMACO), Sudair Pharma Company, Jamjoom Pharma, Hikma Pharmaceuticals PLC (Saudi Operations), Pfizer Scientific and Technical Office – Saudi Arabia, Novartis Saudi Arabia, Gulf Pharmaceutical Industries (Julphar Saudi Arabia), Saudi Chemical Company Holding – Pharma Sector (including AJA Pharma), Arabio – The Saudi Arabian Japanese Pharmaceutical Company Ltd., Avalon Pharma, Jamjoom Medical Solutions (Biologics and Injectables Focus) contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Saudi Arabian recombinant protein therapeutics CDMO market appears promising, driven by increasing investments in healthcare infrastructure and a growing focus on personalized medicine. As the government continues to support biotechnology initiatives, the market is expected to attract more international players, enhancing competition and innovation. Additionally, the rising prevalence of chronic diseases will likely sustain demand for biologics, positioning the CDMO sector for significant growth in the coming years.
| Segment | Sub-Segments |
|---|---|
| By Service Type | Contract Development (Cell Line, Process & Analytical Development) Contract Manufacturing (Clinical & Commercial) Fill–Finish & Packaging Others (Regulatory, Tech Transfer, CMC Consulting) |
| By Molecule / Product Type | Monoclonal Antibodies Recombinant Therapeutic Proteins (Non?mAb) Recombinant Vaccines Hormones & Growth Factors Enzymes & Cytokines Others (Fusion Proteins, Blood Factors, etc.) |
| By Expression System / Technology | Mammalian Cell Culture (e.g., CHO) Microbial (E. coli and Other Bacteria) Yeast (e.g., S. cerevisiae, Pichia) Other Systems (Insect, Plant, Cell?free) |
| By Stage of Development | Pre?clinical Clinical (Phase I–III) Commercial |
| By End-User | Global Pharmaceutical Companies Biotechnology Firms / Start-ups Local & Regional Pharmaceutical Manufacturers Academic & Research Institutions Others |
| By Therapeutic Area | Oncology Metabolic & Endocrine Disorders (e.g., Diabetes) Immunological & Autoimmune Disorders Infectious Diseases Hematology and Others |
| By Region | Central Region (including Riyadh) Western Region (including Jeddah, Makkah, Madinah) Eastern Region (including Dammam, Al Khobar) Northern & Southern Regions |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Biopharmaceutical Development | 100 | R&D Directors, Product Managers |
| Manufacturing Operations | 80 | Operations Managers, Quality Assurance Heads |
| Regulatory Affairs | 60 | Regulatory Affairs Specialists, Compliance Officers |
| Market Access Strategies | 70 | Market Access Managers, Health Economists |
| Healthcare Provider Insights | 90 | Healthcare Professionals, Pharmacists |
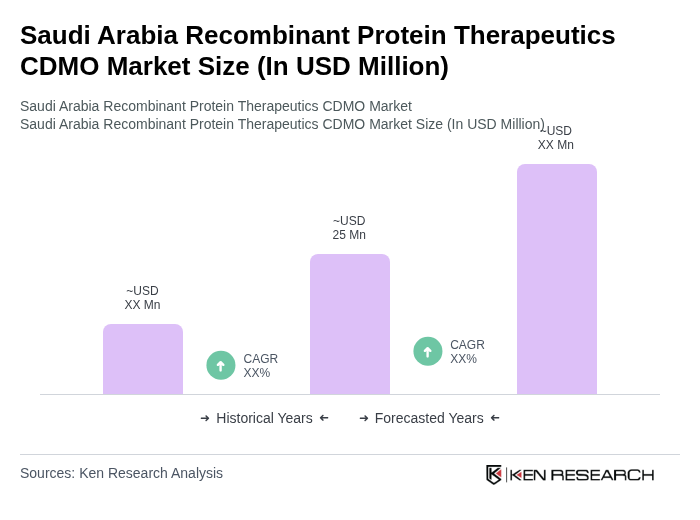
The Saudi Arabia Recombinant Protein Therapeutics CDMO Market is valued at approximately USD 25 million, reflecting a five-year historical analysis. This growth is driven by increasing demand for biologics and advancements in biotechnology.