Region:Middle East
Author(s):Shubham
Product Code:KRAD5438
Pages:90
Published On:December 2025

By Product Type:The product type segmentation includes various devices used in sinus dilation procedures. The subsegments are as follows:
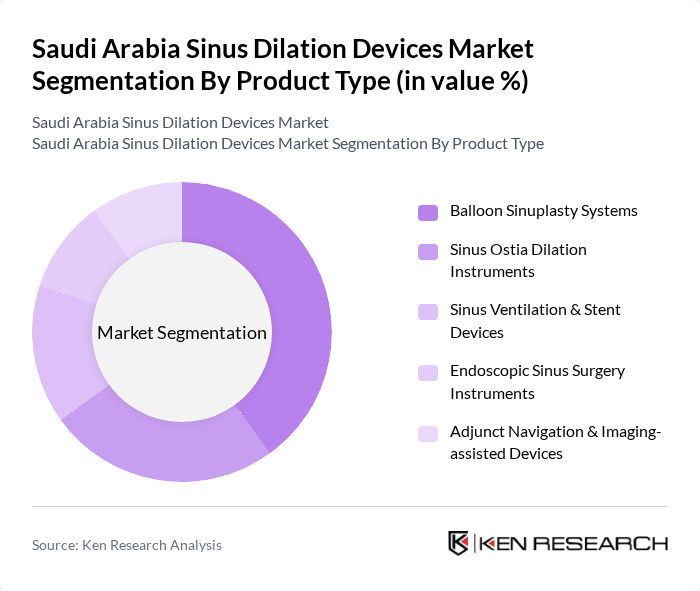
The Balloon Sinuplasty Systems subsegment is currently dominating the market due to their effectiveness in treating chronic sinusitis with minimal invasiveness, reflecting global practice where balloon systems account for a leading share of sinus dilation revenues. These systems are preferred by both patients and healthcare providers for their ability to provide quick recovery times, preservation of mucosal tissue, and reduced postoperative complications compared with traditional functional endoscopic sinus surgery. The increasing awareness among patients and ENT specialists regarding the benefits of balloon sinuplasty, including its suitability for office?based or day?surgery settings, is further driving its adoption, making it the leading product type in the Saudi Arabian market.
By Procedure Setting:The procedure setting segmentation categorizes the environments where sinus dilation procedures are performed. The subsegments are as follows:
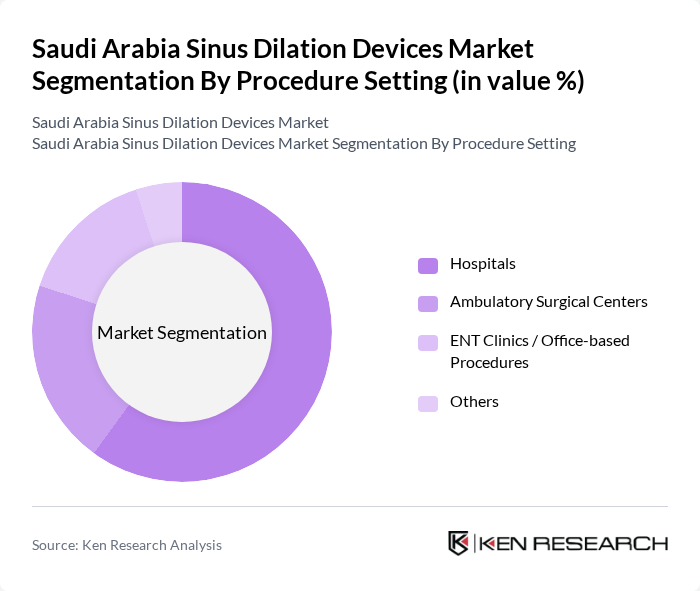
Hospitals are the leading procedure setting for sinus dilation due to their comprehensive facilities and access to advanced medical technologies, consistent with global market patterns where hospitals account for the largest share of sinus dilation device utilization. They provide a wide range of services, including pre-operative assessments, anesthesia support, intra?operative imaging, and post-operative care, which are crucial for successful outcomes, especially in complex chronic rhinosinusitis cases. The presence of specialized ENT departments and trained otolaryngologists within hospitals further enhances their capability to perform complex procedures and to adopt advanced technologies such as navigation and imaging-assisted systems, making them the preferred choice for patients seeking sinus dilation treatments.
The Saudi Arabia Sinus Dilation Devices Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic plc, Acclarent, Inc. (Johnson & Johnson MedTech), Smith & Nephew plc, Olympus Corporation, KARL STORZ SE & Co. KG, Stryker Corporation, Entellus Medical, Inc. (Stryker), SinuSys Corporation, Meril Life Sciences Pvt. Ltd., InnAccel Technologies Pvt. Ltd., B. Braun Melsungen AG, Richard Wolf GmbH, Cook Medical LLC, ConMed Corporation, Karl Kaps GmbH & Co. KG contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Saudi Arabia sinus dilation devices market appears promising, driven by ongoing advancements in medical technology and increasing healthcare investments. As the government continues to enhance healthcare infrastructure, the demand for innovative and effective treatment options is expected to rise. Additionally, the growing trend towards outpatient procedures will likely encourage the adoption of minimally invasive techniques, further propelling market growth. Stakeholders must remain agile to capitalize on these evolving dynamics and address emerging patient needs effectively.
| Segment | Sub-Segments |
|---|---|
| By Product Type | Balloon Sinuplasty Systems Sinus Ostia Dilation Instruments Sinus Ventilation & Stent Devices Endoscopic Sinus Surgery Instruments Adjunct Navigation & Imaging-assisted Devices |
| By Procedure Setting | Hospitals Ambulatory Surgical Centers ENT Clinics / Office-based Procedures Others |
| By Clinical Indication | Chronic Rhinosinusitis with Nasal Polyps (CRSwNP) Chronic Rhinosinusitis without Nasal Polyps (CRSsNP) Recurrent Acute Rhinosinusitis Others |
| By Distribution Channel | Direct Tender to Hospitals & Government Institutions Local Medical Device Distributors Online / E-procurement Platforms Others |
| By Region | Central Region (including Riyadh) Eastern Region (including Dammam/Al Khobar) Western Region (including Jeddah/Makkah/Medina) Southern & Northern Regions |
| By Patient Demographics | Pediatric Patients Adult Patients Geriatric Patients Others |
| By Technology | Manual Devices Powered / Automated Dilation Systems Image-guided & Navigation-enabled Systems |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| ENT Specialists | 100 | Otolaryngologists, Medical Directors |
| Hospital Procurement Managers | 80 | Supply Chain Managers, Purchasing Officers |
| Patients with Sinus Conditions | 120 | Individuals who have undergone sinus dilation |
| Healthcare Policy Makers | 60 | Health Administrators, Policy Analysts |
| Medical Device Distributors | 70 | Sales Representatives, Business Development Managers |
The Saudi Arabia Sinus Dilation Devices Market is valued at approximately USD 30 million, reflecting a five-year historical analysis. This growth is driven by the increasing prevalence of chronic sinusitis and the demand for minimally invasive surgical procedures.