Region:Middle East
Author(s):Geetanshi
Product Code:KRAD4144
Pages:87
Published On:December 2025
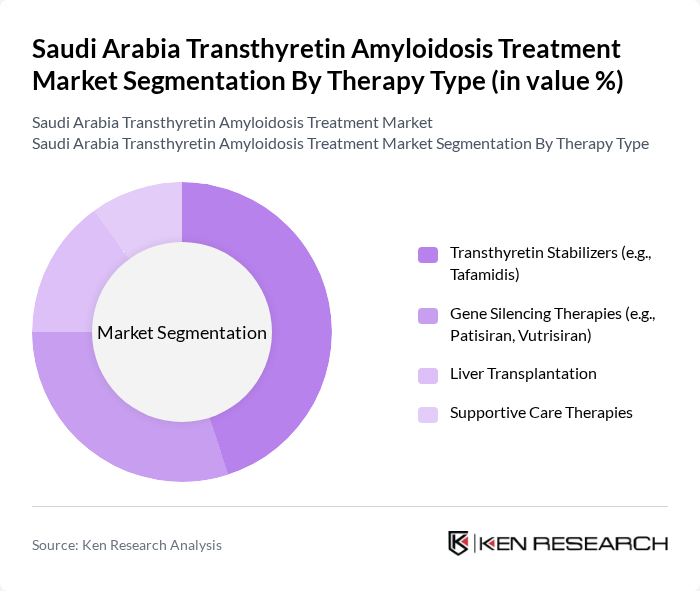
By Therapy Type:The therapy type segmentation includes various treatment modalities for transthyretin amyloidosis, each catering to different patient needs and disease stages. The subsegments include Transthyretin Stabilizers, Gene Silencing Therapies, Liver Transplantation, and Supportive Care Therapies. Among these, Transthyretin Stabilizers, particularly tafamidis, have gained significant traction due to their demonstrated efficacy in stabilizing the transthyretin protein and slowing cardiomyopathy progression in ATTR patients, leading to reduced mortality and hospitalization rates. The increasing adoption of these therapies is driven by favorable Phase III clinical outcomes, inclusion in international heart failure and amyloidosis treatment guidelines, and growing local availability of tafamidis and RNA-interference products through Saudi tertiary centers.
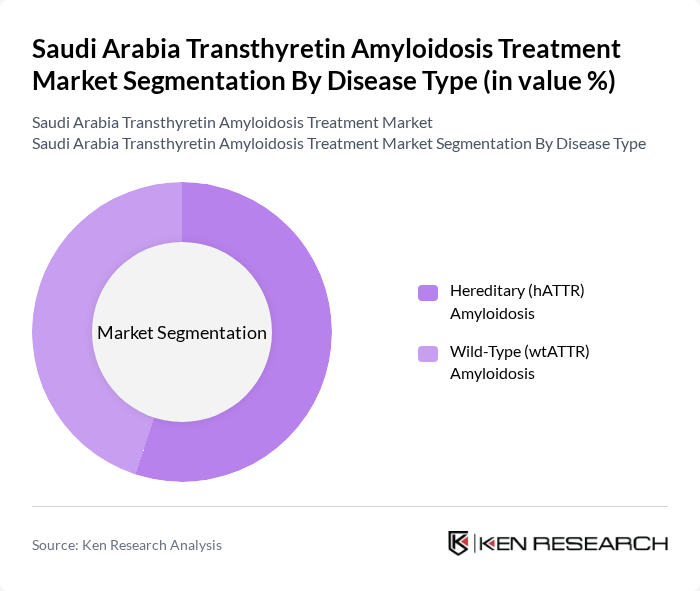
By Disease Type:The disease type segmentation encompasses hereditary (hATTR) and wild-type (wtATTR) amyloidosis. The hereditary form is more prevalent in specific populations with TTR gene variants and is increasingly recognized in Middle Eastern cohorts due to expanding use of genetic testing and family screening. The wild-type form is increasingly recognized in older adults, particularly those presenting with unexplained heart failure with preserved ejection fraction and amyloid cardiomyopathy. The market is primarily driven by rising awareness of these conditions among cardiologists, neurologists, and internists and the availability of targeted therapies such as tafamidis, patisiran, and vutrisiran through specialty hospital pharmacies and rare-disease programs. The hereditary subtype is gaining attention due to its genetic basis, leading to increased cascade screening, earlier diagnosis in affected families, and growing demand for disease-modifying treatment options and long-term follow-up services.
The Saudi Arabia Transthyretin Amyloidosis Treatment Market is characterized by a dynamic mix of regional and international players. Leading participants such as Alnylam Pharmaceuticals, Pfizer Inc., Ionis Pharmaceuticals, Johnson & Johnson (Janssen), Amgen Inc., Novartis AG, Bristol-Myers Squibb Company, Roche Holding AG, Sanofi S.A., Merck & Co., Inc., Regeneron Pharmaceuticals, Inc., GSK plc, Takeda Pharmaceutical Company Limited, Eli Lilly and Company, AbbVie Inc. contribute to innovation, geographic expansion, and service delivery in this space through RNA-based therapeutics, transthyretin stabilizers, cardiology and neurology portfolios, and partnerships with Saudi centers of excellence.
The future of the Transthyretin Amyloidosis treatment market in Saudi Arabia appears promising, driven by ongoing advancements in medical research and technology. As the healthcare infrastructure continues to expand, more patients will gain access to innovative therapies. Additionally, increased collaboration between local and international research organizations is expected to accelerate the development of novel treatments, enhancing patient outcomes and overall market dynamics. The focus on personalized medicine will further tailor therapies to individual patient needs, fostering a more effective treatment landscape.
| Segment | Sub-Segments |
|---|---|
| By Therapy Type | Transthyretin Stabilizers (e.g., Tafamidis) Gene Silencing Therapies (e.g., Patisiran, Vutrisiran) Liver Transplantation Supportive Care Therapies |
| By Disease Type | Hereditary (hATTR) Amyloidosis Wild-Type (wtATTR) Amyloidosis |
| By End-User | Hospitals Specialty Clinics Home Healthcare Others |
| By Treatment Stage | Early Stage Advanced Stage Palliative Care Others |
| By Distribution Channel | Hospital Pharmacies Retail Pharmacies Online Pharmacies Specialty Distributors |
| By Geographic Distribution | Urban Areas Rural Areas Others |
| By Policy Support | Government Subsidies Tax Incentives Research Grants Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Hematology Clinics | 60 | Hematologists, Oncologists |
| Patient Support Groups | 80 | Patients, Caregivers |
| Pharmaceutical Distributors | 45 | Distribution Managers, Sales Representatives |
| Healthcare Policy Makers | 40 | Health Economists, Policy Analysts |
| Clinical Research Organizations | 35 | Clinical Researchers, Trial Coordinators |
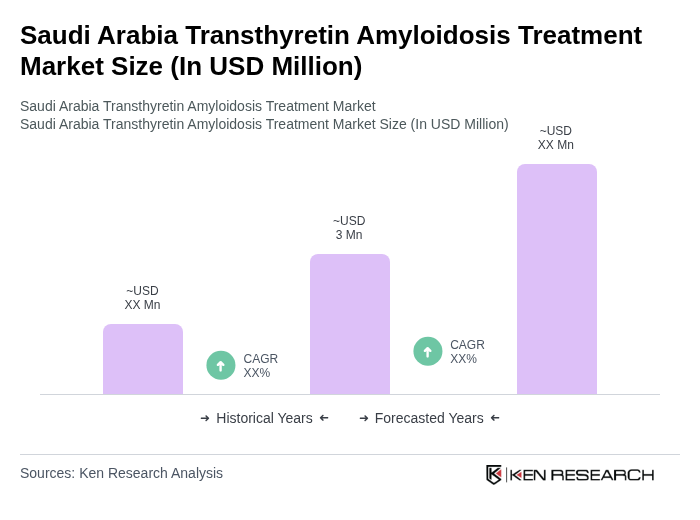
The Saudi Arabia Transthyretin Amyloidosis Treatment Market is valued at approximately USD 3 million, reflecting a five-year historical analysis and recent estimates. This growth is driven by increased case identification and the availability of approved therapies.