Region:Middle East
Author(s):Dev
Product Code:KRAD5265
Pages:81
Published On:December 2025
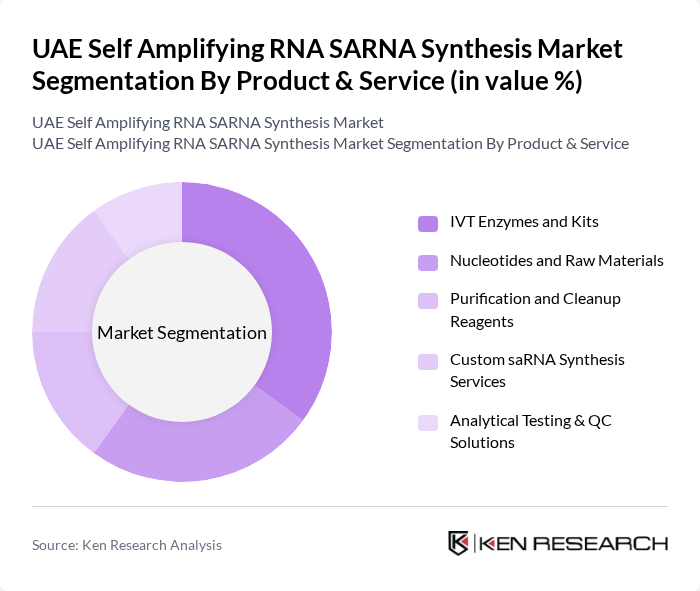
By Product & Service:The product and service segmentation of the SARNA synthesis market includes various components essential for the synthesis process. The leading sub-segment is IVT Enzymes and Kits, which are crucial for the in vitro transcription process, enabling the production of RNA. Nucleotides and Raw Materials follow closely, as they are fundamental building blocks for RNA synthesis. Purification and Cleanup Reagents are also significant, ensuring the quality and efficacy of the synthesized RNA. Custom saRNA Synthesis Services cater to specific client needs, while Analytical Testing & QC Solutions ensure compliance with regulatory standards.
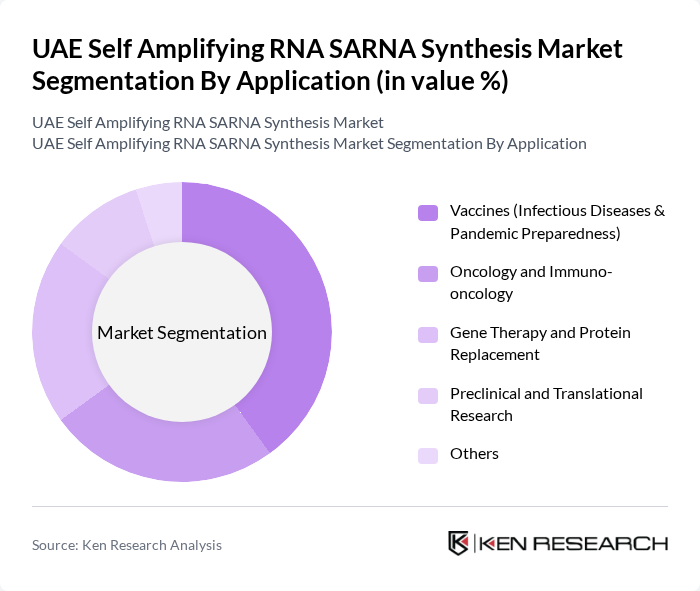
By Application:The application segmentation highlights the various fields where SARNA synthesis is utilized. The most significant application is in Vaccines, particularly for infectious diseases and pandemic preparedness, driven by the urgent need for rapid vaccine development. Oncology and Immuno-oncology applications are also growing, as SARNA technology shows promise in cancer treatment. Gene Therapy and Protein Replacement applications are gaining traction, alongside Preclinical and Translational Research, which are essential for advancing scientific knowledge and therapeutic development.
The UAE Self Amplifying RNA SARNA Synthesis Market is characterized by a dynamic mix of regional and international players. Leading participants such as Moderna, Inc., BioNTech SE, CureVac SE, Arcturus Therapeutics Holdings Inc., AstraZeneca PLC, G42 Healthcare (Abu Dhabi), Abu Dhabi Stem Cells Center (ADSCC), Mubadala Investment Company (Healthcare & Life Sciences), Abu Dhabi Investment Office (ADIO), Dubai Science Park, Thermo Fisher Scientific Inc., Merck KGaA (Merck Life Science), Danaher Corporation (Cytiva & Pall Corporation), Lonza Group AG, Catalent, Inc. contribute to innovation, geographic expansion, and service delivery in this space.
The future of the UAE SARNA synthesis market appears promising, driven by ongoing advancements in RNA technologies and increasing government support for biotechnology. As the demand for mRNA vaccines continues to rise, the market is likely to witness significant growth. Additionally, the integration of artificial intelligence in RNA synthesis processes is expected to enhance efficiency and reduce costs, further propelling market expansion. The focus on personalized medicine and infectious disease preparedness will also shape the future landscape of SARNA technologies in the region.
| Segment | Sub-Segments |
|---|---|
| By Product & Service | IVT Enzymes and Kits Nucleotides and Raw Materials Purification and Cleanup Reagents Custom saRNA Synthesis Services Analytical Testing & QC Solutions |
| By Application | Vaccines (Infectious Diseases & Pandemic Preparedness) Oncology and Immuno-oncology Gene Therapy and Protein Replacement Preclinical and Translational Research Others |
| By End-User | Biopharmaceutical and Vaccine Developers Contract Development & Manufacturing Organizations (CDMOs) Academic and Research Institutes Government & Public Health Agencies Others |
| By Delivery Technology | Lipid Nanoparticle (LNP) Systems Polymer- and Lipid-based Non-viral Carriers Viral and Viral-like Vectors Physical Delivery Methods (e.g., Electroporation) Others |
| By Emirate | Abu Dhabi Dubai Sharjah Northern Emirates (Ajman, Ras Al Khaimah, Fujairah, Umm Al Quwain) |
| By Research & Development Phase | Discovery and Preclinical Clinical Trials (Phase I–III) Commercial-scale Manufacturing Support Others |
| By Funding Source | Federal and Emirate-level Government Programs Sovereign Wealth Funds and Strategic Government Vehicles Private Equity and Venture Capital Corporate and Strategic Investments Multilateral and Non-profit Grants |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| RNA Therapeutics Development | 100 | Biotech Researchers, Clinical Trial Managers |
| Vaccine Production Facilities | 80 | Production Managers, Quality Assurance Officers |
| Academic Research Institutions | 70 | Principal Investigators, Lab Directors |
| Regulatory Affairs in Biotechnology | 60 | Regulatory Affairs Specialists, Compliance Managers |
| Healthcare Policy Makers | 50 | Health Economists, Policy Analysts |
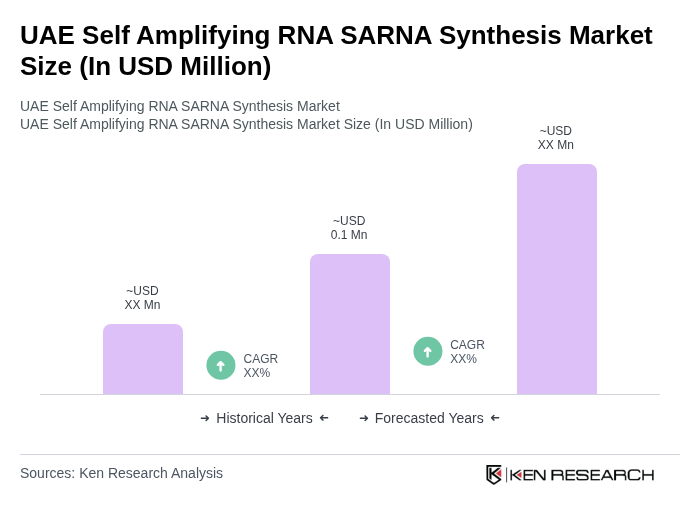
The UAE Self Amplifying RNA SARNA Synthesis Market is valued at approximately USD 0.1 million, reflecting a growing interest in innovative vaccine technologies and biotechnology investments in the region.