Region:Europe
Author(s):Dev
Product Code:KRAB6511
Pages:89
Published On:October 2025
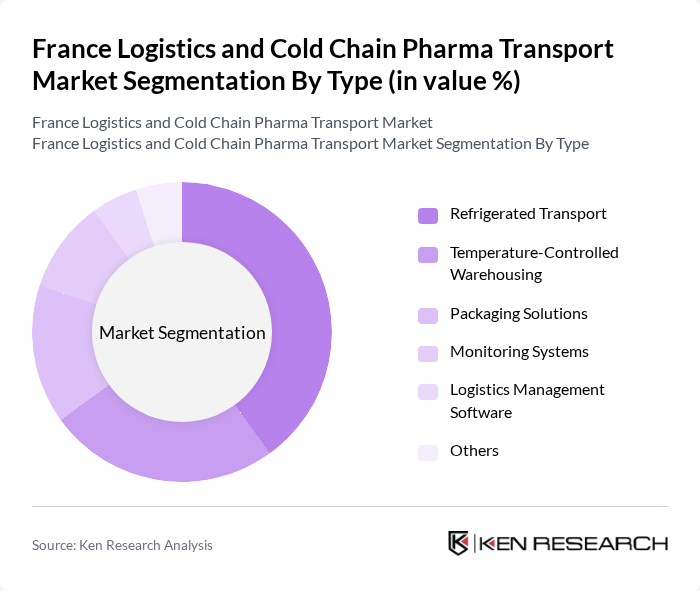
By Type:The market is segmented into various types, including Refrigerated Transport, Temperature-Controlled Warehousing, Packaging Solutions, Monitoring Systems, Logistics Management Software, and Others. Among these, Refrigerated Transport is the leading segment due to the critical need for maintaining specific temperature ranges during the transportation of pharmaceuticals. The increasing focus on supply chain efficiency and product safety drives the demand for advanced refrigerated transport solutions.
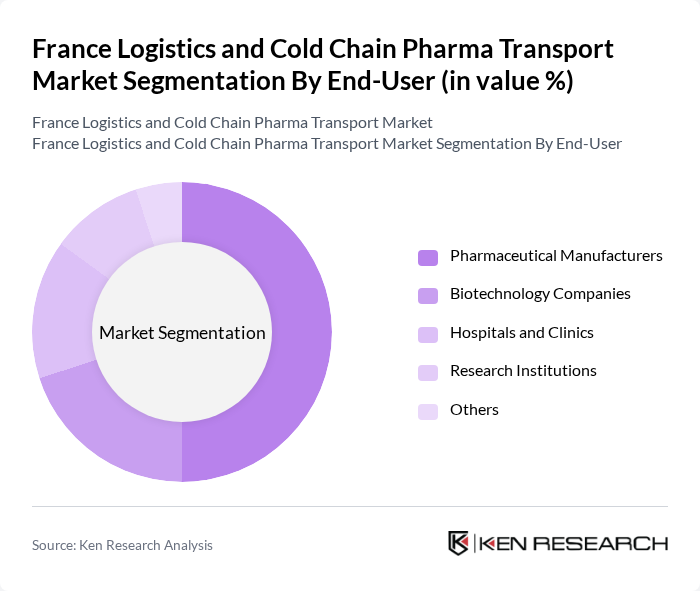
By End-User:The end-user segmentation includes Pharmaceutical Manufacturers, Biotechnology Companies, Hospitals and Clinics, Research Institutions, and Others. Pharmaceutical Manufacturers dominate this segment, driven by the need for reliable logistics solutions to transport sensitive products. The increasing production of biologics and specialty pharmaceuticals further fuels the demand for cold chain logistics tailored to the pharmaceutical industry.
The France Logistics and Cold Chain Pharma Transport Market is characterized by a dynamic mix of regional and international players. Leading participants such as DHL Supply Chain, Kuehne + Nagel, DB Schenker, UPS Healthcare, FedEx, Geodis, XPO Logistics, Cardinal Health, Thermo Fisher Scientific, Agility Logistics, World Courier, CEVA Logistics, Panalpina, Maersk, DSV Panalpina contribute to innovation, geographic expansion, and service delivery in this space.
The future of the logistics and cold chain pharma transport market in France appears promising, driven by technological innovations and regulatory compliance. As the demand for biopharmaceuticals continues to rise, logistics providers are expected to invest in advanced cold chain solutions, enhancing efficiency and reliability. Additionally, the focus on sustainability will likely lead to the adoption of eco-friendly practices, ensuring that the industry meets both regulatory standards and consumer expectations for environmentally responsible operations.
| Segment | Sub-Segments |
|---|---|
| By Type | Refrigerated Transport Temperature-Controlled Warehousing Packaging Solutions Monitoring Systems Logistics Management Software Others |
| By End-User | Pharmaceutical Manufacturers Biotechnology Companies Hospitals and Clinics Research Institutions Others |
| By Distribution Mode | Road Transport Air Transport Rail Transport Sea Transport Others |
| By Packaging Type | Insulated Containers Refrigerated Pallets Active Temperature-Controlled Packaging Passive Temperature-Controlled Packaging Others |
| By Service Type | Transportation Services Warehousing Services Value-Added Services Consulting Services Others |
| By Temperature Range | Controlled Room Temperature (CRT) Refrigerated (2-8°C) Frozen (-20°C and below) Others |
| By Compliance Standards | EU GDP Compliance ISO Standards WHO Guidelines Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Pharmaceutical Cold Chain Providers | 100 | Logistics Managers, Operations Directors |
| Healthcare Institutions | 80 | Pharmacy Directors, Supply Chain Coordinators |
| Regulatory Compliance Experts | 50 | Quality Assurance Managers, Regulatory Affairs Specialists |
| Temperature-Controlled Storage Facilities | 70 | Facility Managers, Operations Supervisors |
| Pharmaceutical Manufacturers | 90 | Procurement Officers, Logistics Coordinators |
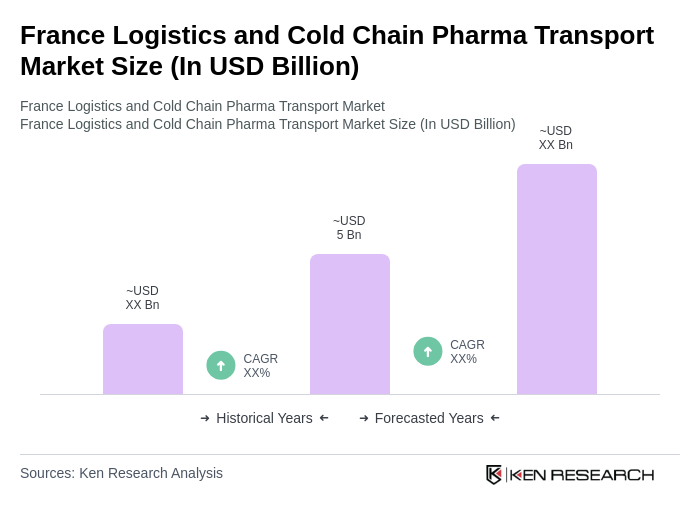
The France Logistics and Cold Chain Pharma Transport Market is valued at approximately USD 5 billion, driven by the increasing demand for temperature-sensitive pharmaceuticals and the expansion of the biopharmaceutical sector.