Region:Global
Author(s):Dev
Product Code:KRAD5269
Pages:99
Published On:December 2025
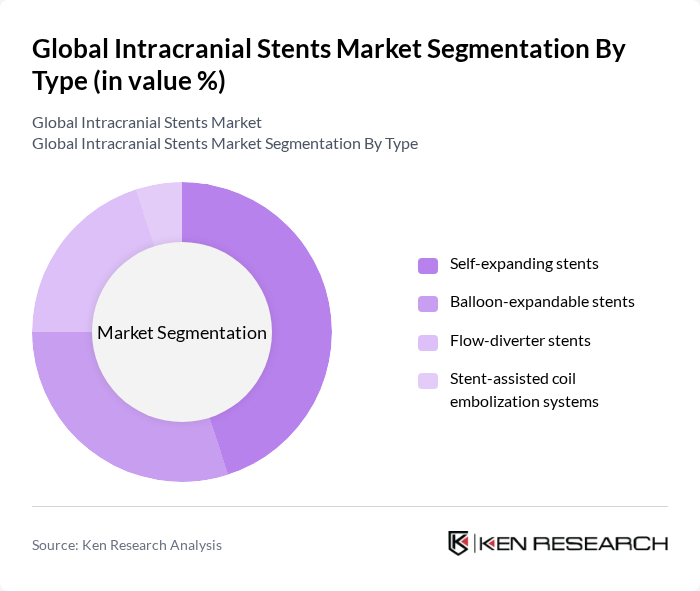
By Type:The market is segmented into various types of stents, including self-expanding stents, balloon-expandable stents, flow-diverter stents, and stent-assisted coil embolization systems. Among these, self-expanding stents are gaining traction due to their adaptability to the vessel's shape and reduced risk of complications. Balloon-expandable stents are also popular for their ease of use and effectiveness in treating specific conditions. The flow-diverter stents are increasingly preferred for complex aneurysms, while stent-assisted coil embolization systems are utilized in specific cases where traditional methods may not suffice.
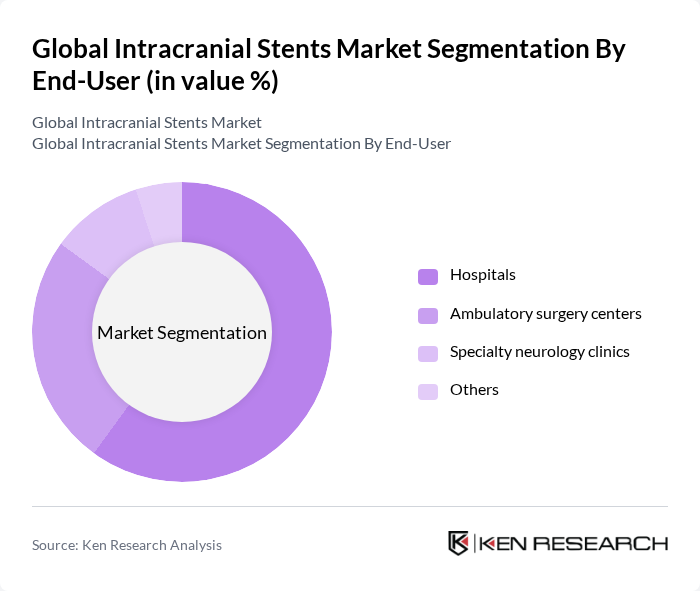
By End-User:The end-user segmentation includes hospitals, ambulatory surgery centers, specialty neurology clinics, and others. Hospitals are the primary end-users due to their comprehensive facilities and access to advanced medical technologies. Ambulatory surgery centers are gaining popularity for their cost-effectiveness and efficiency in performing minimally invasive procedures. Specialty neurology clinics are also emerging as significant players, providing targeted care for neurological conditions.
The Global Intracranial Stents Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic plc, Stryker Corporation, MicroVention, Inc. (Terumo Corporation), Cerenovus (Johnson & Johnson MedTech), Boston Scientific Corporation, Abbott Laboratories, MicroPort Scientific Corporation, Balt Group, Phenox GmbH, Penumbra, Inc., Cook Medical LLC, B. Braun Melsungen AG, Asahi Intecc Co., Ltd., Acandis GmbH, Obex Medical Ltd. contribute to innovation, geographic expansion, and service delivery in this space.
The future of the intracranial stents market appears promising, driven by ongoing advancements in medical technology and an increasing focus on patient-centered care. As healthcare providers continue to embrace minimally invasive procedures, the demand for innovative stent solutions is expected to rise. Additionally, the integration of digital health technologies will enhance patient monitoring and outcomes, further propelling market growth. The emphasis on personalized medicine will also shape the development of tailored stent solutions, ensuring better treatment efficacy for diverse patient populations.
| Segment | Sub-Segments |
|---|---|
| By Type | Self-expanding stents Balloon-expandable stents Flow-diverter stents Stent-assisted coil embolization systems |
| By End-User | Hospitals Ambulatory surgery centers Specialty neurology clinics Others |
| By Material | Nitinol stents Cobalt-chromium stents Stainless steel stents Polymer-coated and drug-eluting stents |
| By Delivery Method | Endovascular (transfemoral) delivery Transradial endovascular delivery Others |
| By Application | Intracranial stenosis Brain aneurysm (including flow diversion) Ischemic stroke (stent-assisted interventions) Others |
| By Region | North America Europe Asia-Pacific Latin America Middle East & Africa |
| By Distribution Channel | Direct sales to hospitals and ASC Medical device distributors Group purchasing organizations (GPOs) Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Neurosurgery Departments | 100 | Neurosurgeons, Department Heads |
| Interventional Radiology Units | 80 | Interventional Radiologists, Clinical Coordinators |
| Hospital Procurement Teams | 70 | Procurement Managers, Supply Chain Analysts |
| Medical Device Distributors | 60 | Sales Representatives, Regional Managers |
| Clinical Research Organizations | 50 | Clinical Researchers, Data Analysts |
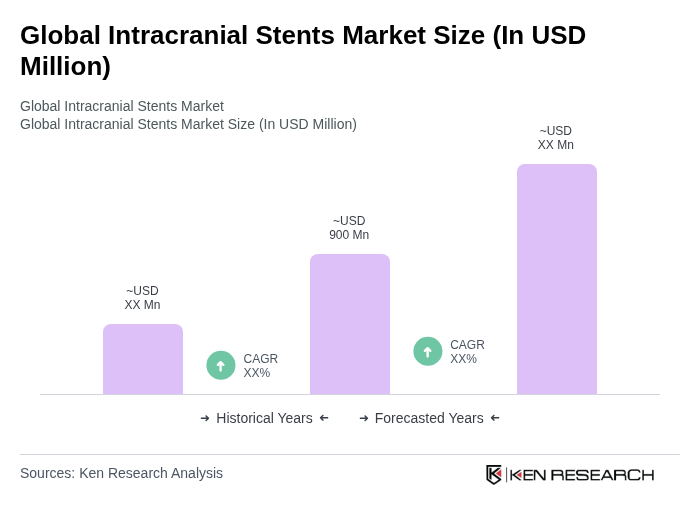
The Global Intracranial Stents Market is valued at approximately USD 900 million, reflecting a significant growth driven by the rising prevalence of neurological disorders and advancements in stent technology.