Region:Asia
Author(s):Geetanshi
Product Code:KRAD1110
Pages:91
Published On:November 2025
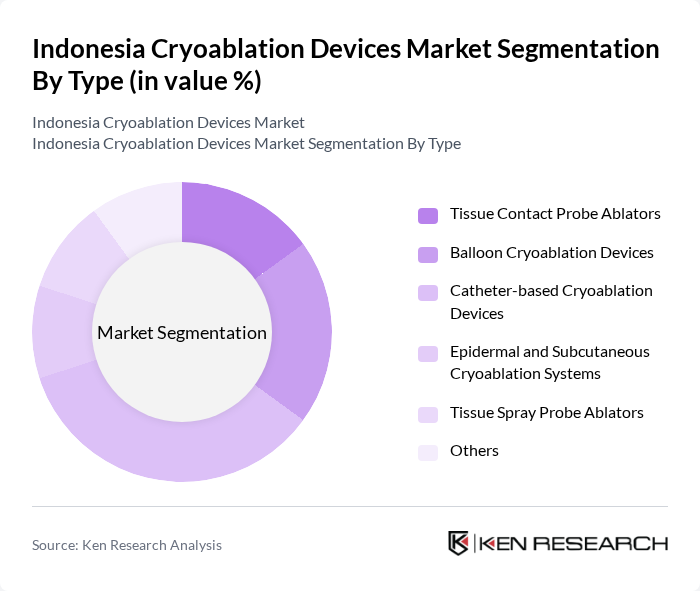
By Type:The cryoablation devices market can be segmented into various types, including Tissue Contact Probe Ablators, Balloon Cryoablation Devices, Catheter-based Cryoablation Devices, Epidermal and Subcutaneous Cryoablation Systems, Tissue Spray Probe Ablators, and Others. Among these, Catheter-based Cryoablation Devices are leading the market due to their effectiveness in treating cardiac arrhythmias and their growing acceptance among healthcare professionals.
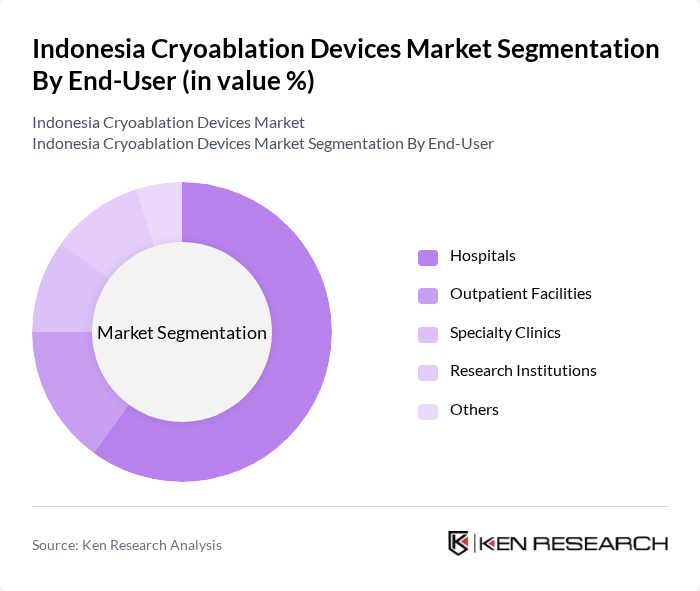
By End-User:The end-user segmentation includes Hospitals, Outpatient Facilities, Specialty Clinics, Research Institutions, and Others. Hospitals are the dominant end-user segment, primarily due to their comprehensive facilities and the availability of specialized medical staff, which facilitates the adoption of advanced cryoablation technologies.
The Indonesia Cryoablation Devices Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic Plc, Boston Scientific Corporation, Abbott Laboratories, Johnson & Johnson (Biosense Webster), Biotronik SE & Co. KG, AtriCure Inc., CryoCath Technologies (now part of Medtronic), Stereotaxis Inc., AngioDynamics Inc., ConMed Corporation, Merit Medical Systems Inc., Philips Healthcare, Siemens Healthineers, GE Healthcare, Terumo Corporation, IceCure Medical Ltd., HealthTronics Inc., Metrum Cryoflex Sp. z o.o., CooperSurgical Inc., CPSI Biotech (Cell Preservation Services Inc.) contribute to innovation, geographic expansion, and service delivery in this space.
The future of the cryoablation devices market in Indonesia appears promising, driven by ongoing advancements in medical technology and increasing healthcare investments. As the government prioritizes healthcare infrastructure development, more facilities are expected to adopt cryoablation techniques. Additionally, the growing trend towards outpatient procedures will likely enhance the market's appeal, as patients seek less invasive treatment options. These factors combined suggest a robust growth trajectory for the cryoablation devices market in the coming years.
| Segment | Sub-Segments |
|---|---|
| By Type | Tissue Contact Probe Ablators Balloon Cryoablation Devices Catheter-based Cryoablation Devices Epidermal and Subcutaneous Cryoablation Systems Tissue Spray Probe Ablators Others |
| By End-User | Hospitals Outpatient Facilities Specialty Clinics Research Institutions Others |
| By Application | Cardiac Arrhythmia (including Atrial Fibrillation) Lung Cancer Liver Cancer Breast Cancer Kidney Cancer Prostate Cancer Pain Management Others |
| By Distribution Channel | Direct Sales Distributors Online Sales Others |
| By Region | Java Sumatra Bali Kalimantan Sulawesi Others |
| By Patient Demographics | Age Group Gender Socioeconomic Status Others |
| By Technology | Cryoablation Technology Hybrid Technologies Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Cardiology Departments | 100 | Interventional Cardiologists, Cardiology Nurses |
| Oncology Clinics | 80 | Oncologists, Radiation Therapists |
| Pain Management Centers | 50 | Pain Specialists, Anesthesiologists |
| Medical Device Distributors | 40 | Sales Managers, Product Specialists |
| Healthcare Procurement Departments | 60 | Procurement Officers, Supply Chain Managers |
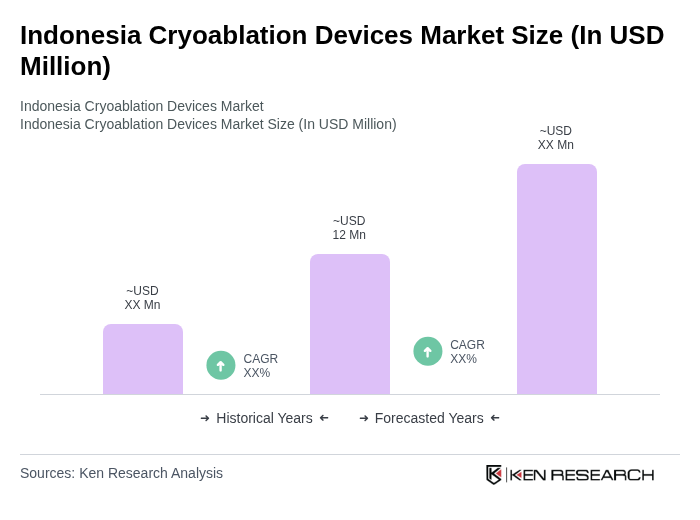
The Indonesia Cryoablation Devices Market is valued at approximately USD 12 million, driven by the rising prevalence of cancer and cardiovascular diseases, advancements in minimally invasive technology, and increased healthcare expenditure.