Region:Europe
Author(s):Geetanshi
Product Code:KRAA0117
Pages:93
Published On:August 2025
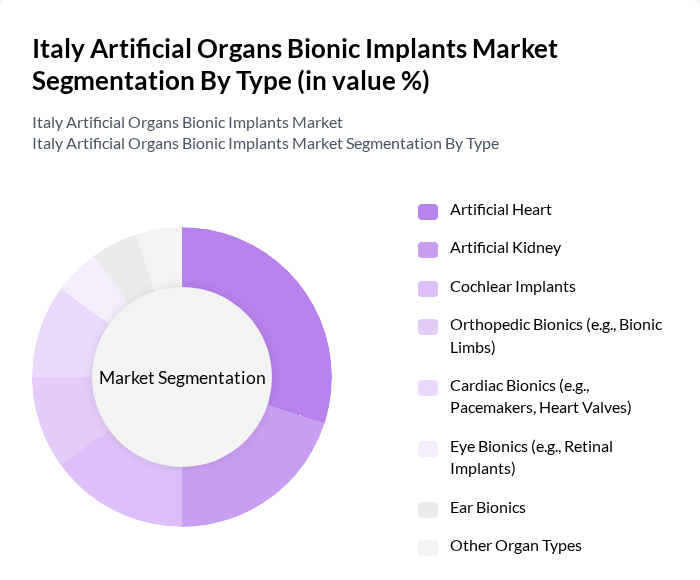
By Type:The market is segmented into various types of artificial organs and bionic implants, including Artificial Heart, Artificial Kidney, Cochlear Implants, Orthopedic Bionics (e.g., Bionic Limbs), Cardiac Bionics (e.g., Pacemakers, Heart Valves), Eye Bionics (e.g., Retinal Implants), Ear Bionics, and Other Organ Types. Among these, the Artificial Heart segment is currently leading the market due to the rising incidence of cardiovascular diseases and advancements in heart implant technologies.
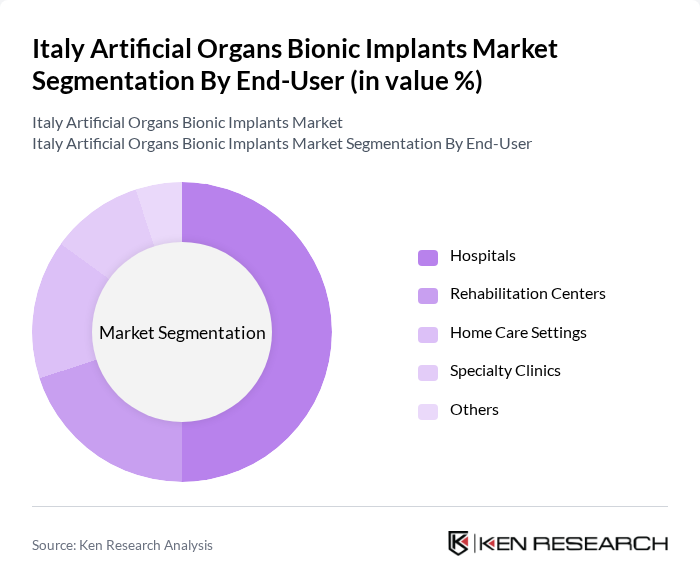
By End-User:The market is segmented by end-users, including Hospitals, Rehabilitation Centers, Home Care Settings, Specialty Clinics, and Others. Hospitals are the leading end-user segment, driven by the high volume of surgical procedures and the availability of advanced medical technologies. The increasing number of patients requiring surgical interventions for chronic conditions further supports the growth of this segment.
The Italy Artificial Organs Bionic Implants Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic plc, Abbott Laboratories, Boston Scientific Corporation, Johnson & Johnson (DePuy Synthes), Stryker Corporation, Zimmer Biomet Holdings, Inc., Cochlear Limited, Biotronik SE & Co. KG, Ottobock SE & Co. KGaA, Ossur hf, Orthofix Medical Inc., LivaNova PLC, Nevro Corp., SynCardia Systems LLC, Sorin Group (now part of LivaNova PLC) contribute to innovation, geographic expansion, and service delivery in this space.
The future of the bionic implants market in Italy appears promising, driven by ongoing technological advancements and an increasing focus on personalized medicine. As healthcare providers adopt more innovative solutions, the integration of artificial intelligence in bionic devices is expected to enhance patient monitoring and outcomes. Furthermore, the expansion of healthcare infrastructure will facilitate better access to these advanced medical technologies, ultimately improving the quality of care for patients requiring organ replacements.
| Segment | Sub-Segments |
|---|---|
| By Type | Artificial Heart Artificial Kidney Cochlear Implants Orthopedic Bionics (e.g., Bionic Limbs) Cardiac Bionics (e.g., Pacemakers, Heart Valves) Eye Bionics (e.g., Retinal Implants) Ear Bionics Other Organ Types |
| By End-User | Hospitals Rehabilitation Centers Home Care Settings Specialty Clinics Others |
| By Application | Cardiovascular Applications Orthopedic Applications Neurological Applications Sensory Applications (Hearing, Vision) Others |
| By Material | Biocompatible Materials Metals Polymers Ceramics Others |
| By Distribution Channel | Direct Sales Online Sales Distributors Others |
| By Region | Northern Italy Central Italy Southern Italy Others |
| By Patient Demographics | Pediatric Patients Adult Patients Geriatric Patients Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Orthopedic Surgeons | 60 | Surgeons specializing in joint replacements and bionic implants |
| Hospital Procurement Managers | 50 | Managers responsible for purchasing medical devices and implants |
| Patients with Bionic Implants | 40 | Individuals who have received bionic implants in the last 5 years |
| Medical Device Distributors | 40 | Distributors involved in the supply chain of bionic implants |
| Healthcare Policy Makers | 40 | Officials involved in healthcare regulations and policies |
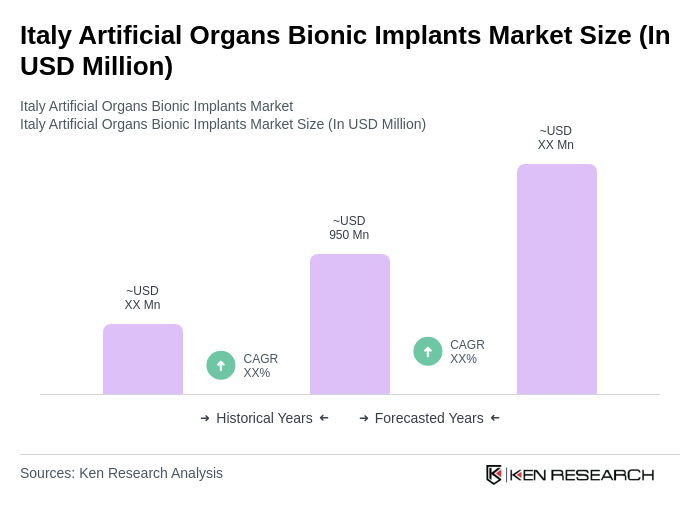
The Italy Artificial Organs Bionic Implants Market is valued at approximately USD 950 million, reflecting significant growth driven by advancements in medical technology, an aging population, and the increasing prevalence of chronic diseases requiring organ replacements and advanced prosthetics.