Region:Middle East
Author(s):Dev
Product Code:KRAD6345
Pages:87
Published On:December 2025
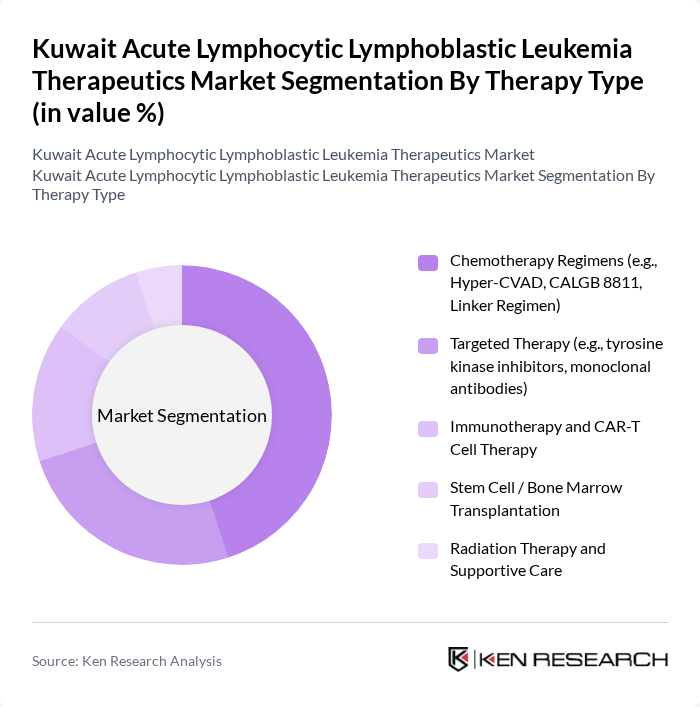
By Therapy Type:The market is segmented into various therapy types, including chemotherapy regimens, targeted therapy, immunotherapy, stem cell transplantation, and radiation therapy. Chemotherapy regimens remain the backbone of treatment and are the most widely used due to their established efficacy, standardized multi?phase protocols, and inclusion in international and regional ALL treatment guidelines. Targeted therapies, such as tyrosine kinase inhibitors and monoclonal antibodies for specific genetic subtypes and relapsed/refractory disease, are gaining traction as they offer more personalized and often better?tolerated treatment options. Immunotherapy, particularly bispecific antibodies and CAR?T cell therapy, is emerging as a transformative approach in treating ALL, especially in heavily pretreated patients, and is increasingly being accessed in Kuwait through referral pathways and international centers.
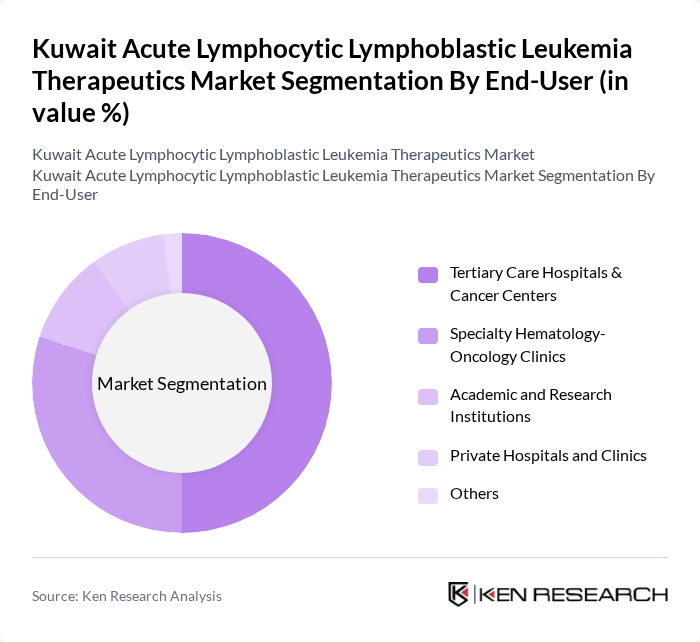
By End-User:The end-user segmentation includes tertiary care hospitals, specialty hematology-oncology clinics, academic institutions, and private hospitals. Tertiary care hospitals and cancer centers dominate the market due to their comprehensive treatment capabilities, multidisciplinary tumor boards, and ability to deliver high?complexity therapies such as intensive chemotherapy, targeted agents, and hematopoietic stem cell transplantation. Specialty clinics are also significant as they provide focused care and long?term follow?up for hematological malignancies, while academic and research institutions support clinical trials and protocol?driven care, and private hospitals increasingly participate in diagnostics and supportive oncology services for a growing patient population.
The Kuwait Acute Lymphocytic Lymphoblastic Leukemia Therapeutics Market is characterized by a dynamic mix of regional and international players. Leading participants such as Novartis AG, Amgen Inc., Pfizer Inc., F. Hoffmann-La Roche Ltd, Takeda Pharmaceutical Company Limited, Bristol Myers Squibb Company, Sanofi S.A., Merck & Co., Inc., Janssen Biotech, Inc. (Johnson & Johnson), Gilead Sciences, Inc., AbbVie Inc., AstraZeneca PLC, Bayer AG, Eli Lilly and Company, Kuwait Saudi Pharmaceutical Industries Company (KSPICO) contribute to innovation, geographic expansion, portfolio diversification in hematology-oncology, and service delivery in this space through the availability of chemotherapy backbones, targeted agents, and immunotherapies used in ALL treatment pathways.
The future of the Kuwait Acute Lymphocytic Lymphoblastic Leukemia therapeutics market appears promising, driven by ongoing advancements in treatment options and increased healthcare investments. The government’s commitment to enhancing healthcare infrastructure and fostering collaborations with international research institutions will likely lead to improved patient outcomes. Additionally, the growing focus on personalized medicine and digital health solutions is expected to transform treatment paradigms, making therapies more effective and accessible for patients in Kuwait.
| Segment | Sub-Segments |
|---|---|
| By Therapy Type | Chemotherapy Regimens (e.g., Hyper-CVAD, CALGB 8811, Linker Regimen) Targeted Therapy (e.g., tyrosine kinase inhibitors, monoclonal antibodies) Immunotherapy and CAR-T Cell Therapy Stem Cell / Bone Marrow Transplantation Radiation Therapy and Supportive Care |
| By End-User | Tertiary Care Hospitals & Cancer Centers Specialty Hematology-Oncology Clinics Academic and Research Institutions Private Hospitals and Clinics Others |
| By Patient Demographics | Pediatric Patients (0–14 Years) Adolescent and Young Adult Patients (15–39 Years) Adult and Geriatric Patients (40+ Years) Others |
| By Treatment Line | First-Line Therapy Second-Line / Salvage Therapy Relapsed / Refractory ALL Therapy Maintenance Therapy |
| By Distribution Channel | Hospital Pharmacies Retail Pharmacies Specialty Pharmacies Others |
| By Route of Administration | Oral Parenteral (Intravenous, Subcutaneous, Intrathecal) Others |
| By Clinical Trial Phase (Pipeline Therapies in Kuwait / GCC) | Phase I Phase II Phase III Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Pediatric ALL Treatment Insights | 45 | Pediatric Oncologists, Child Psychologists |
| Adult ALL Treatment Protocols | 40 | Hematologists, Medical Oncologists |
| Patient Experience and Outcomes | 40 | Patients, Caregivers, Support Group Leaders |
| Pharmaceutical Market Access | 40 | Pharmaceutical Sales Representatives, Market Access Managers |
| Healthcare Policy and Funding | 40 | Health Economists, Policy Makers, Hospital Administrators |
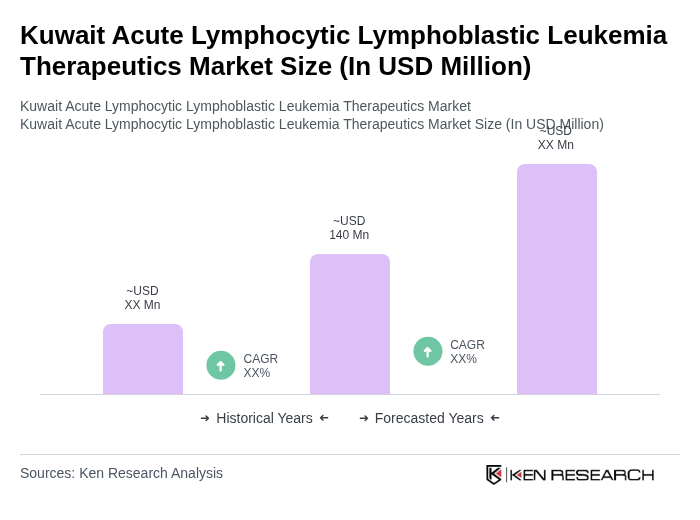
The Kuwait Acute Lymphocytic Lymphoblastic Leukemia Therapeutics Market is valued at approximately USD 140 million, reflecting the country's significant public healthcare spending and the increasing incidence of acute lymphoblastic leukemia (ALL).