Region:Middle East
Author(s):Shubham
Product Code:KRAD3553
Pages:86
Published On:November 2025
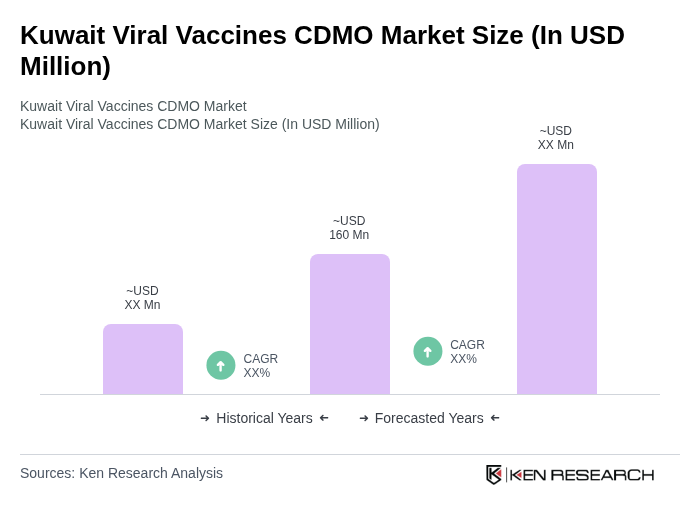
By Type:The market is segmented into Inactivated Vaccines, Live Attenuated Vaccines, Subunit Vaccines, mRNA Vaccines, Viral Vector Vaccines, and Others. Among these, mRNA Vaccines have gained substantial traction due to their rapid development cycles and high efficacy, particularly highlighted during the COVID-19 pandemic. The adoption of mRNA technology continues to rise, driven by its flexibility and scalability for emerging infectious diseases and personalized vaccine applications .
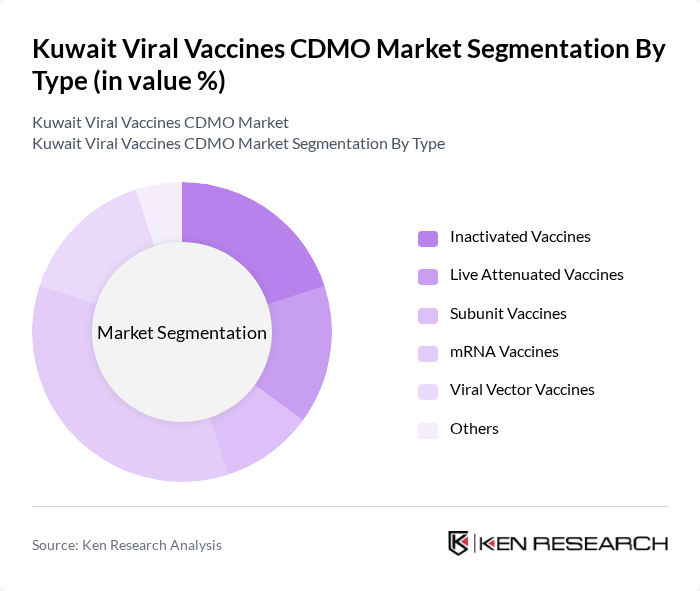
By End-User:The end-user segmentation includes Hospitals, Research Institutions, Pharmaceutical & Biotechnology Companies, Government Health Departments, Contract Research Organizations (CROs), and Others. Hospitals are the leading end-users, supported by the expansion of national vaccination programs and the increasing need for effective disease management solutions. Collaboration between hospitals and vaccine manufacturers remains critical to ensuring timely vaccine access and distribution .
The Kuwait Viral Vaccines CDMO Market is characterized by a dynamic mix of regional and international players. Leading participants such as Kuwait Vaccine Manufacturing Company (KVMC), Gulf Pharmaceutical Industries (Julphar), Agility Life Sciences, Mubarak Al-Kabeer Biopharma, Kuwait Medical Supplies Manufacturing Company (KMSMC), United Pharmaceutical Industries Company (UPIC), Advanced Technology Company (ATC), Global Pharma Kuwait, Al-Sayer Healthcare, Pharmax Pharmaceuticals, Kuwait Institute for Scientific Research (KISR), Kuwait University - Faculty of Medicine, Kuwait Medical Association, Ministry of Health, Kuwait, Kuwait Pharmaceutical Association contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Kuwait Viral Vaccines CDMO market appears promising, driven by increasing investments in healthcare infrastructure and technological advancements. As the government prioritizes healthcare improvements, the demand for innovative vaccine solutions is expected to rise. Additionally, the collaboration between local CDMOs and international partners will likely enhance knowledge transfer and operational efficiencies, positioning Kuwait as a regional hub for vaccine production and distribution in the coming years.
| Segment | Sub-Segments |
|---|---|
| By Type | Inactivated Vaccines Live Attenuated Vaccines Subunit Vaccines mRNA Vaccines Viral Vector Vaccines Others |
| By End-User | Hospitals Research Institutions Pharmaceutical & Biotechnology Companies Government Health Departments Contract Research Organizations (CROs) Others |
| By Distribution Channel | Direct Sales Distributors Online Platforms Others |
| By Application | Preventive Vaccines Therapeutic Vaccines Pandemic Preparedness Vaccines Others |
| By Technology | Recombinant DNA Technology Virus-Like Particle (VLP) Technology Cell Culture-Based Technology Others |
| By Funding Source | Government Funding Private Investments International Grants Public-Private Partnerships Others |
| By Policy Support | Subsidies for Local Production Tax Incentives Research Grants Fast-Track Regulatory Approvals Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Vaccine Manufacturing Facilities | 60 | Production Managers, Quality Assurance Officers |
| Healthcare Providers | 70 | Doctors, Pharmacists, Public Health Officials |
| Regulatory Bodies | 40 | Regulatory Affairs Specialists, Compliance Officers |
| Research Institutions | 50 | Research Scientists, Vaccine Development Experts |
| Distribution Networks | 45 | Logistics Managers, Supply Chain Coordinators |
The Kuwait Viral Vaccines CDMO Market is valued at approximately USD 160 million, reflecting its significant share within the broader Kuwait vaccine market, which generated total revenues of USD 242.7 million.