Region:Middle East
Author(s):Dev
Product Code:KRAC1951
Pages:91
Published On:October 2025
By Type:The market is segmented into various types of vaccines, including Inactivated Vaccines, Live Attenuated Vaccines, Subunit Vaccines, mRNA Vaccines, Viral Vector Vaccines, DNA Vaccines, Recombinant Protein Vaccines, and Others. Among these, mRNA Vaccines have gained significant traction due to their rapid development and effectiveness in combating viral infections, particularly highlighted during the COVID-19 pandemic. The global viral vaccines CDMO market shows that viral vector-based platforms are increasingly adopted for both prophylactic and therapeutic use, driven by high biosafety, quality, and process control requirements, with pharmaceutical companies turning to CDMOs with established viral platforms and BSL-2/3 containment infrastructure.

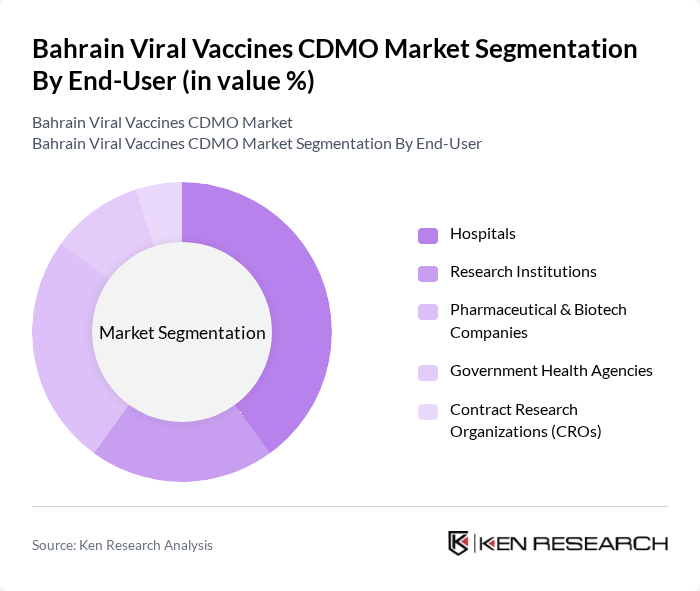
By End-User:The end-user segmentation includes Hospitals, Research Institutions, Pharmaceutical & Biotech Companies, Government Health Agencies, and Contract Research Organizations (CROs). Hospitals are the leading end-users, driven by the increasing need for vaccination programs and the rising prevalence of infectious diseases, which necessitate a robust supply of vaccines. Biopharmaceutical and vaccine developers are increasingly partnering with CDMOs to accelerate time-to-market and reduce operational costs, while government health agencies are expanding their engagement with CDMOs for emergency preparedness and mass vaccination campaigns.
The Bahrain Viral Vaccines CDMO Market is characterized by a dynamic mix of regional and international players. Leading participants such as GSK Vaccines, Pfizer Inc., Moderna, Inc., Sanofi Pasteur, Merck & Co., Inc., Novavax, Inc., AstraZeneca, Johnson & Johnson, Bharat Biotech, Sinovac Biotech, BioNTech SE, Valneva SE, VBI Vaccines Inc., Inovio Pharmaceuticals, Inc., Zydus Cadila, Samsung Biologics, Lonza Group AG, WuXi Biologics, Recipharm AB, Benuvia Operations, Avernus Pharma, Gulf Biotech (Bahrain), Julphar (Gulf Pharmaceutical Industries), Biocon Limited contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Bahrain viral vaccines CDMO market appears promising, driven by increasing government support and a growing emphasis on public health initiatives. As local manufacturing capabilities expand, CDMOs are likely to enhance their production efficiency and innovation. Furthermore, the collaboration between local firms and international research institutions is expected to foster advancements in vaccine technology, positioning Bahrain as a competitive player in the regional biotechnology landscape, particularly in personalized vaccine development.
| Segment | Sub-Segments |
|---|---|
| By Type | Inactivated Vaccines Live Attenuated Vaccines Subunit Vaccines mRNA Vaccines Viral Vector Vaccines DNA Vaccines Recombinant Protein Vaccines Others |
| By End-User | Hospitals Research Institutions Pharmaceutical & Biotech Companies Government Health Agencies Contract Research Organizations (CROs) |
| By Application | Preventive Vaccines Therapeutic Vaccines Travel Vaccines Pediatric Vaccines Adult Vaccines |
| By Distribution Channel | Direct Sales Distributors Online Platforms Government Procurement Programs |
| By Regulatory Compliance Level | WHO Prequalification National Regulatory Approval (NHRA Bahrain) CE Marking GCC-DR Approval |
| By Production Scale | Small Scale Medium Scale Large Scale |
| By Policy Support | Subsidies Tax Exemptions Research Grants Public-Private Partnerships |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Vaccine Manufacturers | 45 | Production Managers, Quality Assurance Officers |
| Healthcare Providers | 65 | Pharmacists, Clinic Administrators |
| Regulatory Bodies | 40 | Policy Makers, Compliance Officers |
| Public Health Officials | 55 | Epidemiologists, Health Program Managers |
| Supply Chain Experts | 50 | Logistics Coordinators, Distribution Managers |
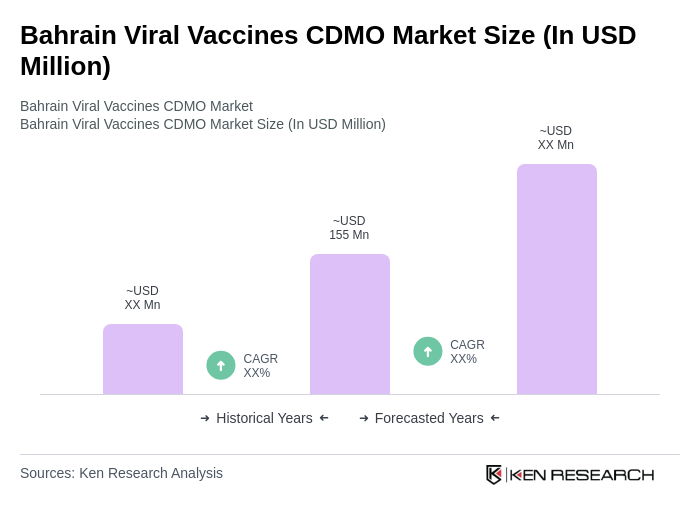
The Bahrain Viral Vaccines CDMO Market is valued at approximately USD 155 million, reflecting significant growth driven by increasing demand for viral vaccines and investments in research and development within the region.