Region:Middle East
Author(s):Shubham
Product Code:KRAD5542
Pages:85
Published On:December 2025
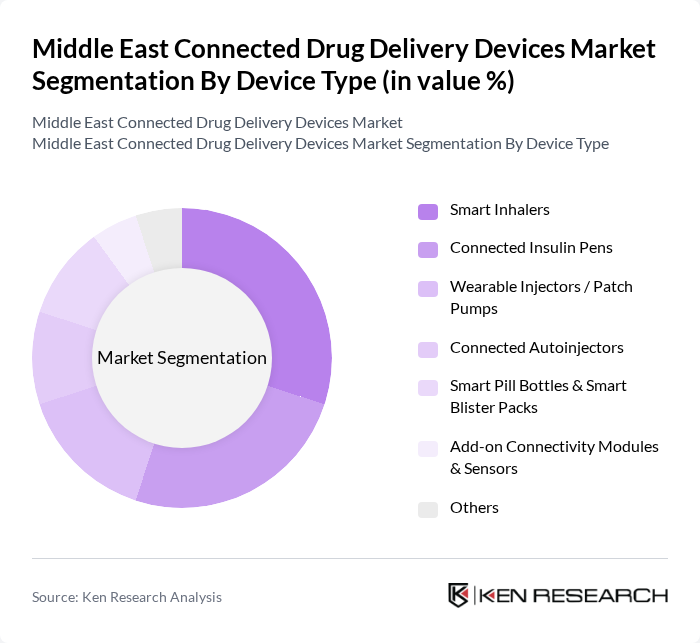
By Device Type:The device type segmentation includes various innovative technologies that cater to different patient needs. The subsegments are Smart Inhalers, Connected Insulin Pens, Wearable Injectors / Patch Pumps, Connected Autoinjectors, Smart Pill Bottles & Smart Blister Packs, Add-on Connectivity Modules & Sensors, and Others. This structure aligns with the main connected drug delivery categories discussed in global market analyses, where smart inhalers, connected insulin devices, wearable injectors, add?on sensors and smart packaging are identified as key product families. Among these, Smart Inhalers are gaining traction due to the increasing prevalence of respiratory diseases such as asthma and COPD in Gulf countries and Israel, and the need for effective adherence and technique management solutions that can record inhalation events and support physician review.
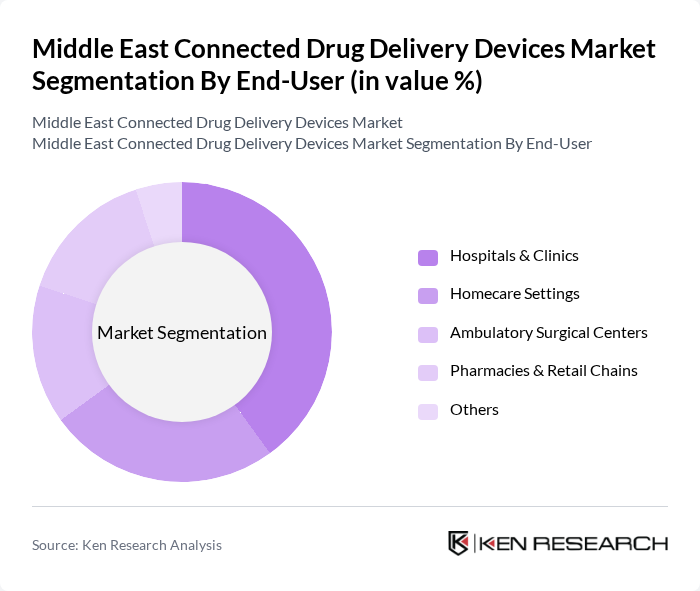
By End-User:The end-user segmentation encompasses various healthcare settings where connected drug delivery devices are utilized. This includes Hospitals & Clinics, Homecare Settings, Ambulatory Surgical Centers, Pharmacies & Retail Chains, and Others. Hospitals & Clinics are the leading end-users due to their need for advanced medication management systems, integration with electronic medical records and e?prescribing platforms, and the adoption of connected devices to support chronic disease programs and value?based care models. Homecare Settings are also expanding in importance as connected injectors, insulin devices and smart packaging enable remote monitoring and self?administration outside traditional clinical environments.
The Middle East Connected Drug Delivery Devices Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic plc, Abbott Laboratories, F. Hoffmann?La Roche Ltd, Johnson & Johnson (Janssen / J&J MedTech), Becton, Dickinson and Company (BD), Insulet Corporation, Novo Nordisk A/S, Sanofi, Teva Pharmaceutical Industries Ltd., AstraZeneca plc, GSK plc, BIOCORP Production SA, Ypsomed AG, Enable Injections, Inc., West Pharmaceutical Services, Inc. contribute to innovation, geographic expansion, and service delivery in this space, through portfolios that include connected insulin delivery systems, wearable injectors, smart inhalers, and add?on connectivity modules.
The future of the Middle East connected drug delivery devices market appears promising, driven by technological advancements and a growing emphasis on patient-centric care. As healthcare systems increasingly adopt digital health solutions, the integration of artificial intelligence and real-time data analytics will enhance the efficiency of drug delivery systems. Furthermore, the expansion of telehealth services will facilitate remote patient monitoring, creating a conducive environment for innovative drug delivery solutions to thrive in the region.
| Segment | Sub-Segments |
|---|---|
| By Device Type | Smart Inhalers Connected Insulin Pens Wearable Injectors / Patch Pumps Connected Autoinjectors Smart Pill Bottles & Smart Blister Packs Add?on Connectivity Modules & Sensors Others |
| By End-User | Hospitals & Clinics Homecare Settings Ambulatory Surgical Centers Pharmacies & Retail Chains Others |
| By Therapeutic Area | Diabetes Management Respiratory Disorders (Asthma, COPD, etc.) Cardiovascular & Hypertension Oncology & Autoimmune Disorders Pain Management & Others |
| By Connectivity Technology | Bluetooth Low Energy (BLE) Wi?Fi NFC Cellular / LPWAN (3G/4G/5G, NB?IoT) Integrated Cloud?Connected Platforms Others |
| By Region | GCC Countries (Saudi Arabia, UAE, Qatar, Kuwait, Oman, Bahrain) Levant (Jordan, Lebanon, Iraq, etc.) North Africa (Egypt and Rest of North Africa) Rest of Middle East |
| By Distribution Channel | Direct Sales to Providers & Payers Online & eHealth Platforms Medical Device & Pharma Distributors Hospital & Retail Pharmacies Others |
| By User Demographics | Age Group Gender Socioeconomic Status Digital Literacy & Technology Adoption Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Pharmaceutical Manufacturers | 60 | Product Managers, R&D Directors |
| Healthcare Providers | 80 | Doctors, Nurses, Pharmacists |
| Patients Using Connected Devices | 90 | Chronic Disease Patients, General Users |
| Regulatory Bodies | 40 | Health Policy Makers, Compliance Officers |
| Technology Developers | 70 | Software Engineers, Product Developers |
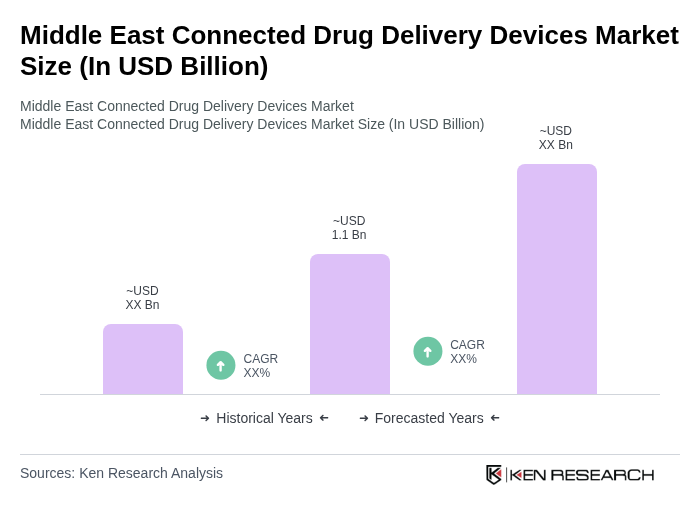
The Middle East Connected Drug Delivery Devices Market is valued at approximately USD 1.1 billion, reflecting a significant growth trend driven by the increasing prevalence of chronic diseases and advancements in digital health technologies.