Region:Middle East
Author(s):Dev
Product Code:KRAB7787
Pages:91
Published On:October 2025
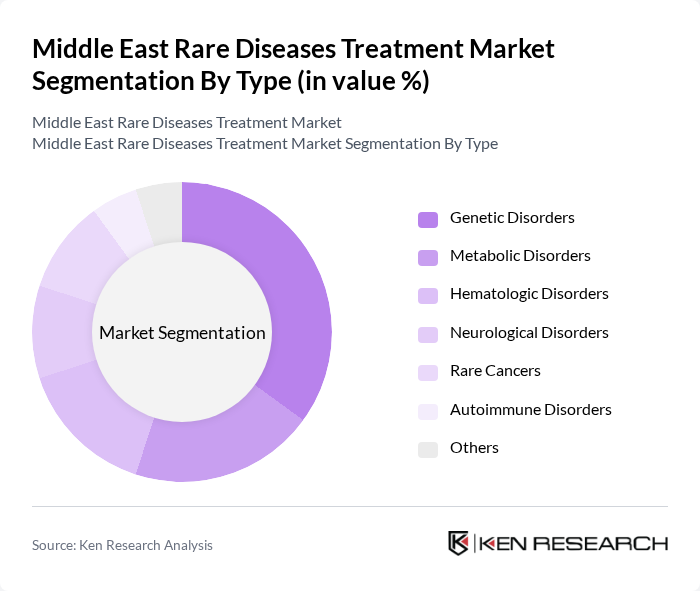
By Type:The market is segmented into various types of rare diseases, including genetic disorders, metabolic disorders, hematologic disorders, neurological disorders, rare cancers, autoimmune disorders, and others. Among these, genetic disorders are currently the most significant segment due to the increasing incidence of inherited conditions and the growing availability of targeted therapies. The rise in genetic testing and awareness of genetic disorders has also contributed to the dominance of this segment.
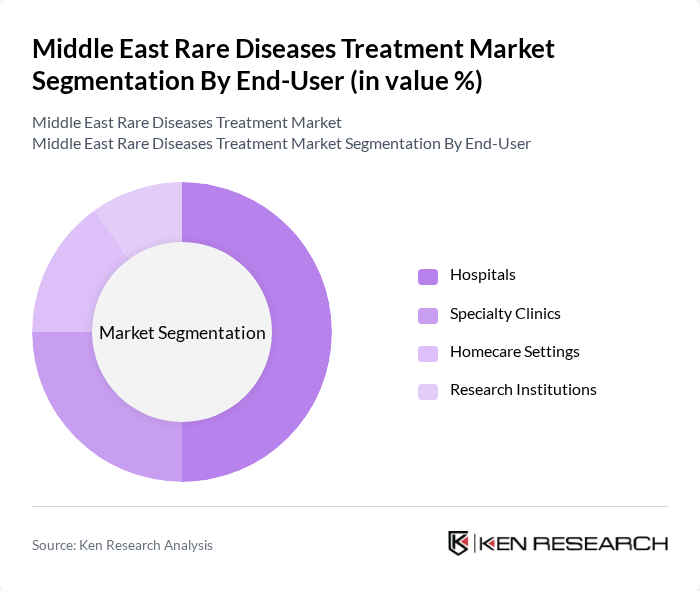
By End-User:The end-user segmentation includes hospitals, specialty clinics, homecare settings, and research institutions. Hospitals are the leading end-user segment, primarily due to their capacity to provide comprehensive care and access to advanced treatment options for patients with rare diseases. The increasing number of specialized hospitals and the integration of rare disease treatment protocols into standard care practices have further solidified this segment's dominance.
The Middle East Rare Diseases Treatment Market is characterized by a dynamic mix of regional and international players. Leading participants such as Novartis AG, Sanofi S.A., Pfizer Inc., Roche Holding AG, Amgen Inc., Vertex Pharmaceuticals Incorporated, BioMarin Pharmaceutical Inc., Takeda Pharmaceutical Company Limited, Alexion Pharmaceuticals, Inc., GSK plc, AbbVie Inc., Regeneron Pharmaceuticals, Inc., Eli Lilly and Company, Merck & Co., Inc., Sobi AB contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Middle East rare diseases treatment market is poised for transformative growth, driven by technological advancements and increased collaboration among stakeholders. The integration of digital health solutions and telemedicine is expected to enhance patient access to specialized care, while the rise of personalized medicine will lead to more effective treatment options. Additionally, ongoing government support and funding initiatives will further stimulate research and development, paving the way for innovative therapies that address the unique needs of rare disease patients in the region.
| Segment | Sub-Segments |
|---|---|
| By Type | Genetic Disorders Metabolic Disorders Hematologic Disorders Neurological Disorders Rare Cancers Autoimmune Disorders Others |
| By End-User | Hospitals Specialty Clinics Homecare Settings Research Institutions |
| By Distribution Channel | Direct Sales Distributors Online Pharmacies Retail Pharmacies |
| By Region | GCC Countries Levant Region North Africa Others |
| By Treatment Type | Pharmacological Treatments Gene Therapy Enzyme Replacement Therapy Stem Cell Therapy |
| By Patient Demographics | Pediatric Patients Adult Patients Geriatric Patients |
| By Research and Development Stage | Preclinical Stage Clinical Stage Marketed Products |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Healthcare Providers in Rare Disease Treatment | 150 | Specialists, General Practitioners, and Nurses |
| Pharmaceutical Companies Focused on Rare Diseases | 100 | Product Managers, Market Access Directors |
| Patient Advocacy Groups and Organizations | 80 | Advocacy Leaders, Patient Representatives |
| Healthcare Policy Makers and Regulators | 60 | Health Ministry Officials, Regulatory Affairs Managers |
| Research Institutions and Academic Experts | 70 | Researchers, Professors, and Clinicians |
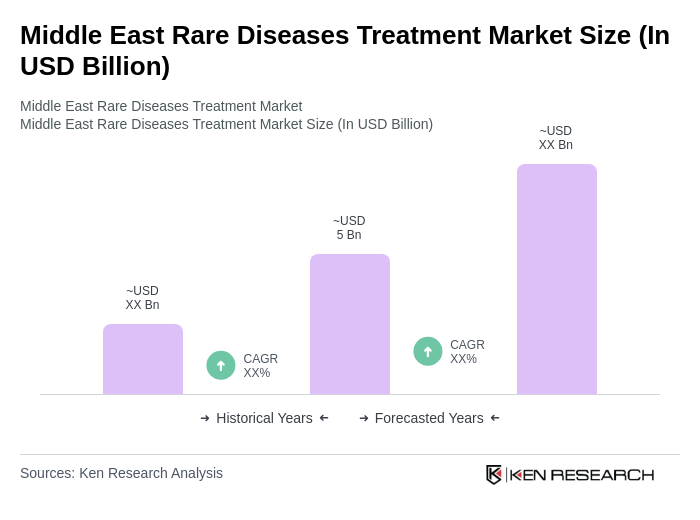
The Middle East Rare Diseases Treatment Market is valued at approximately USD 5 billion, reflecting a significant growth driven by increased awareness, advancements in biotechnology, and rising prevalence of genetic disorders in the region.