Region:Middle East
Author(s):Geetanshi
Product Code:KRAB8115
Pages:81
Published On:October 2025
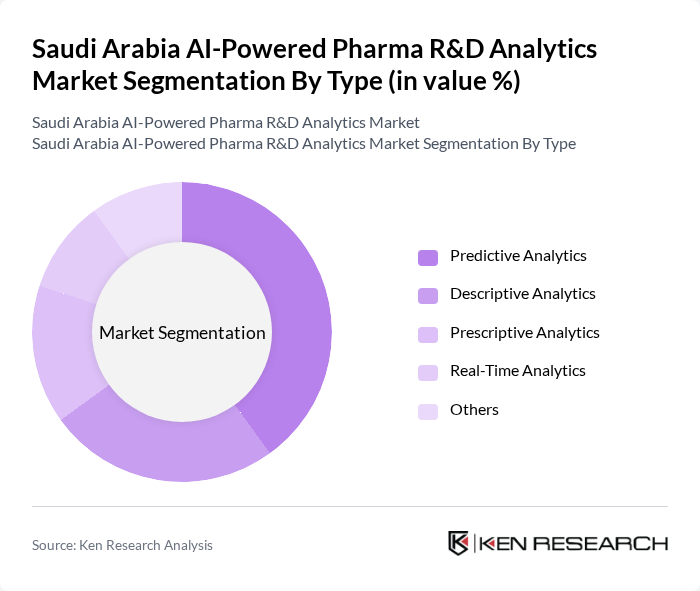
By Type:The market is segmented into various types of analytics, including predictive analytics, descriptive analytics, prescriptive analytics, real-time analytics, and others. Among these, predictive analytics is currently the leading sub-segment due to its ability to forecast outcomes and trends, which is crucial for drug development and clinical trial success. The demand for predictive analytics is driven by its effectiveness in identifying potential drug candidates and optimizing clinical trial designs, making it a preferred choice for pharmaceutical companies.
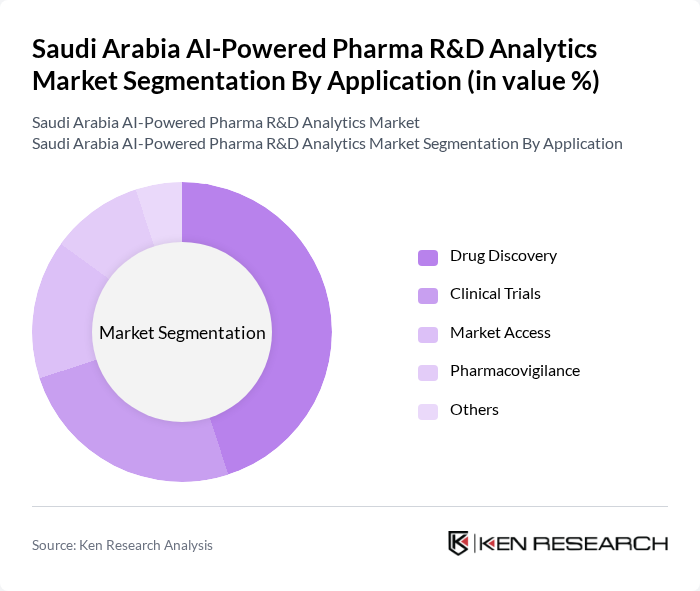
By Application:The applications of AI-powered analytics in the pharmaceutical sector include drug discovery, clinical trials, market access, pharmacovigilance, and others. Drug discovery is the dominant application area, as it leverages AI to analyze vast datasets for identifying new drug candidates and predicting their efficacy. The increasing complexity of drug development processes and the need for faster time-to-market are driving the adoption of AI in drug discovery, making it a critical focus for pharmaceutical companies.
The Saudi Arabia AI-Powered Pharma R&D Analytics Market is characterized by a dynamic mix of regional and international players. Leading participants such as Novartis AG, Pfizer Inc., Roche Holding AG, Merck & Co., Inc., Johnson & Johnson, AstraZeneca PLC, Sanofi S.A., GSK (GlaxoSmithKline) PLC, Eli Lilly and Company, Amgen Inc., Bayer AG, AbbVie Inc., Biogen Inc., Takeda Pharmaceutical Company Limited, Teva Pharmaceutical Industries Ltd. contribute to innovation, geographic expansion, and service delivery in this space.
The future of the AI-powered pharma R&D analytics market in Saudi Arabia appears promising, driven by technological advancements and supportive government policies. As healthcare investments increase, the integration of AI with big data analytics is expected to enhance drug development processes. Furthermore, the shift towards decentralized clinical trials will likely facilitate more efficient data collection and analysis, ultimately leading to faster drug approvals and improved patient outcomes in the region.
| Segment | Sub-Segments |
|---|---|
| By Type | Predictive Analytics Descriptive Analytics Prescriptive Analytics Real-Time Analytics Others |
| By Application | Drug Discovery Clinical Trials Market Access Pharmacovigilance Others |
| By End-User | Pharmaceutical Companies Biotechnology Firms Research Institutions Contract Research Organizations (CROs) Others |
| By Region | Central Region Eastern Region Western Region Southern Region Others |
| By Sales Channel | Direct Sales Distributors Online Platforms Others |
| By Investment Source | Private Investments Government Funding Venture Capital Others |
| By Policy Support | Government Grants Tax Incentives Research Funding Programs Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Pharmaceutical R&D Managers | 100 | R&D Directors, Project Managers |
| AI Technology Implementers | 80 | Data Scientists, AI Engineers |
| Regulatory Affairs Specialists | 60 | Compliance Officers, Regulatory Managers |
| Healthcare Policy Makers | 50 | Government Officials, Policy Analysts |
| Market Analysts in Pharma | 70 | Market Research Analysts, Business Development Managers |
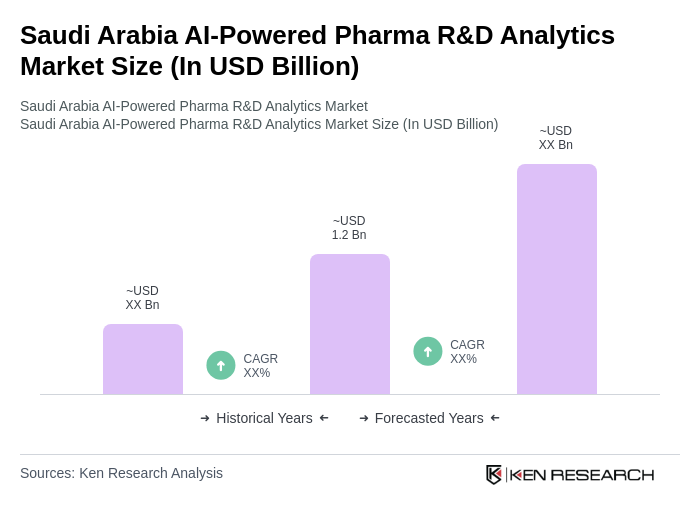
The Saudi Arabia AI-Powered Pharma R&D Analytics Market is valued at approximately USD 1.2 billion, reflecting significant growth driven by the adoption of AI technologies in pharmaceutical research and development, enhancing efficiency and reducing costs in drug discovery and clinical trials.