Region:Middle East
Author(s):Shubham
Product Code:KRAD3671
Pages:84
Published On:November 2025
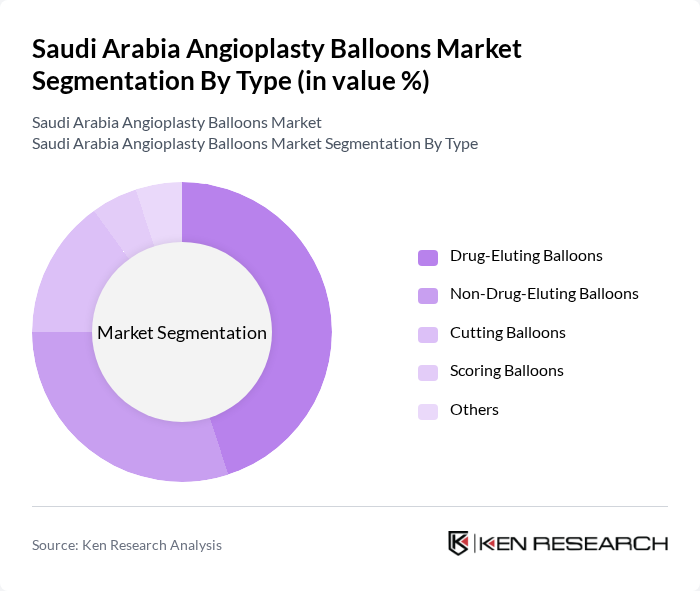
By Type:The market is segmented into various types of angioplasty balloons, including Drug-Eluting Balloons, Non-Drug-Eluting Balloons, Cutting Balloons, Scoring Balloons, and Others. Among these, Drug-Eluting Balloons are gaining significant traction due to their ability to reduce restenosis rates and improve patient outcomes. The increasing preference for minimally invasive procedures is also contributing to the growth of Non-Drug-Eluting Balloons. Cutting and Scoring Balloons are witnessing steady adoption for complex coronary interventions, while the Others segment includes specialty balloons for niche applications .
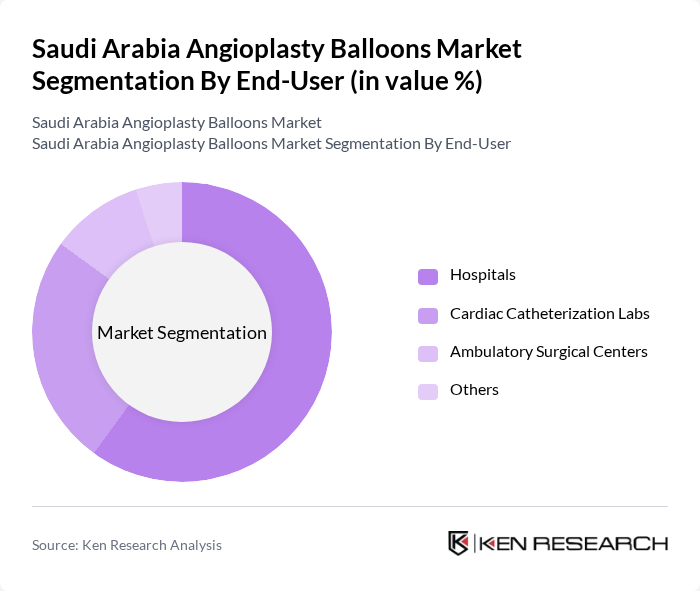
By End-User:The end-user segmentation includes Hospitals, Cardiac Catheterization Labs, Ambulatory Surgical Centers, and Others. Hospitals are the primary end-users due to their comprehensive facilities and specialized staff for performing angioplasty procedures. The increasing number of cardiac surgeries in hospitals is driving the demand for angioplasty balloons. Cardiac Catheterization Labs are also witnessing growth due to the rising volume of diagnostic and interventional procedures, while Ambulatory Surgical Centers are expanding their offerings to include minimally invasive cardiac interventions .
The Saudi Arabia Angioplasty Balloons Market is characterized by a dynamic mix of regional and international players. Leading participants such as Boston Scientific Corporation, Medtronic plc, Abbott Laboratories, B. Braun Melsungen AG, Terumo Corporation, Cordis Corporation, Cook Medical, Asahi Intecc Co., Ltd., Philips Healthcare, Biotronik SE & Co. KG, Stryker Corporation, Cardinal Health, Merit Medical Systems, Inc., Medline Industries, LP, Teleflex Incorporated contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Saudi Arabia angioplasty balloons market appears promising, driven by ongoing advancements in medical technology and increasing healthcare investments. As the government continues to enhance healthcare infrastructure, the accessibility of angioplasty procedures is expected to improve significantly. Additionally, the rising awareness of cardiovascular health among the population will likely lead to earlier diagnosis and treatment, further propelling market growth. The integration of telemedicine and digital health solutions will also play a crucial role in optimizing patient care and follow-up.
| Segment | Sub-Segments |
|---|---|
| By Type | Drug-Eluting Balloons Non-Drug-Eluting Balloons Cutting Balloons Scoring Balloons Others |
| By End-User | Hospitals Cardiac Catheterization Labs Ambulatory Surgical Centers Others |
| By Application | Coronary Angioplasty Peripheral Angioplasty Others |
| By Material | Polyurethane Nylon Others |
| By Distribution Channel | Direct Sales Distributors Online Sales Others |
| By Region | Central Region Eastern Region Western Region Southern Region |
| By Price Range | Low Price Range Mid Price Range High Price Range Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Cardiologists in Major Hospitals | 100 | Interventional Cardiologists, Electrophysiologists |
| Healthcare Procurement Managers | 80 | Procurement Officers, Supply Chain Managers |
| Medical Device Distributors | 60 | Sales Managers, Distribution Executives |
| Clinical Research Coordinators | 50 | Clinical Researchers, Trial Managers |
| Healthcare Policy Makers | 40 | Health Economists, Policy Analysts |
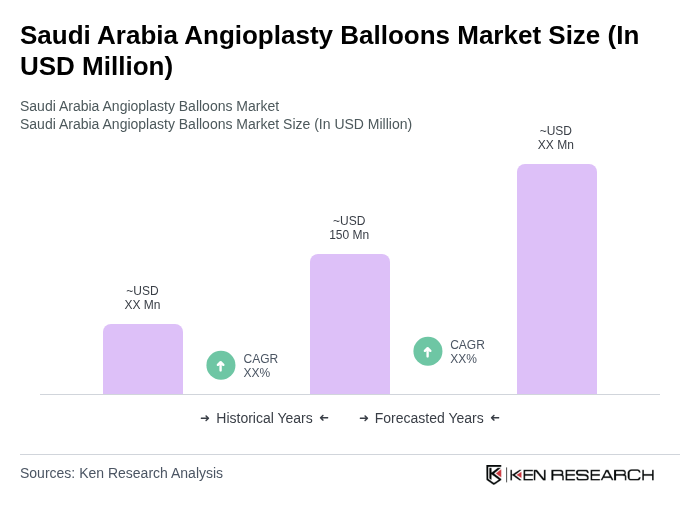
The Saudi Arabia Angioplasty Balloons Market is valued at approximately USD 150 million, driven by the rising prevalence of cardiovascular diseases, advancements in medical technology, and an aging population requiring more angioplasty procedures.