Region:Middle East
Author(s):Rebecca
Product Code:KRAC2579
Pages:91
Published On:October 2025
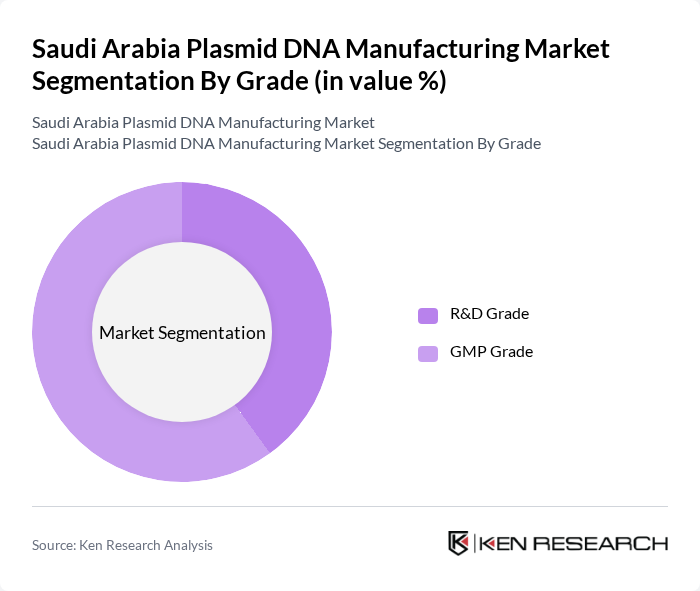
By Grade:The market is segmented into R&D Grade and GMP Grade. R&D Grade plasmid DNA is primarily used for research purposes, while GMP Grade is essential for clinical applications and commercial production. The GMP Grade segment is the dominant segment, reflecting the increasing demand for high-quality plasmid DNA in therapeutic applications and the regulatory emphasis on GMP compliance for clinical and commercial use.
By Application:The applications of plasmid DNA include Gene Therapy, Vaccine Development, Diagnostic Applications, and Others. Gene Therapy is the leading application segment, driven by the increasing focus on personalized medicine and the development of innovative therapies for genetic disorders. Vaccine Development remains significant, especially following recent global health challenges and the expansion of biopharmaceutical partnerships. Diagnostic Applications and other uses continue to grow as biomedical research intensifies in Saudi Arabia.

The Saudi Arabia Plasmid DNA Manufacturing Market is characterized by a dynamic mix of regional and international players. Leading participants such as SaudiVax, BioVac, Theraclion, Tamer Group, and MilliporeSigma (Supporting SaudiVax) contribute to innovation, geographic expansion, and service delivery in this space. Strategic partnerships and collaborations, such as MilliporeSigma's support for SaudiVax in Halal vaccine development, are common and drive market expansion.
The future of the plasmid DNA manufacturing market in Saudi Arabia appears promising, driven by increasing investments in biotechnology and a growing focus on personalized medicine. As the government continues to support research and development initiatives, local manufacturers are expected to enhance their production capabilities. Additionally, collaborations with international biotech firms will likely facilitate knowledge transfer and technology adoption, further strengthening the market's position in the region.
| Segment | Sub-Segments |
|---|---|
| By Grade | R&D Grade GMP Grade |
| By Application | Gene Therapy Vaccine Development Diagnostic Applications Others |
| By End-User | Pharmaceutical Companies Research Institutions Biotechnology Firms Others |
| By Distribution Channel | Direct Sales Distributors Online Sales Others |
| By Region | Central Region Eastern Region Western Region Southern Region |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Gene Therapy Applications | 60 | Biotech Researchers, Clinical Trial Managers |
| Vaccine Development | 50 | Pharmaceutical Scientists, Regulatory Affairs Specialists |
| Academic Research Institutions | 40 | University Professors, Research Lab Directors |
| Contract Manufacturing Organizations (CMOs) | 45 | Operations Managers, Business Development Executives |
| Healthcare Providers and Hospitals | 55 | Medical Directors, Procurement Officers |
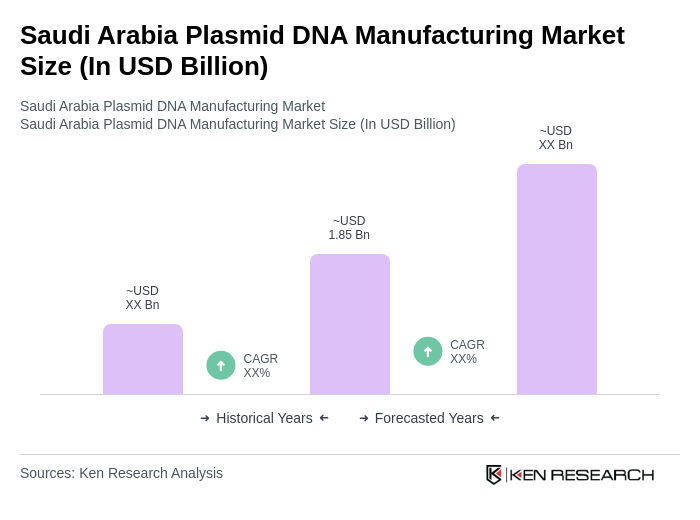
The Saudi Arabia Plasmid DNA Manufacturing Market is valued at approximately USD 1.85 billion, reflecting significant growth driven by increasing demand for plasmid DNA in gene therapy, vaccine development, and advancements in biopharmaceutical manufacturing technologies.