Region:North America
Author(s):Dev
Product Code:KRAD5073
Pages:99
Published On:December 2025
 Devices Market.png)
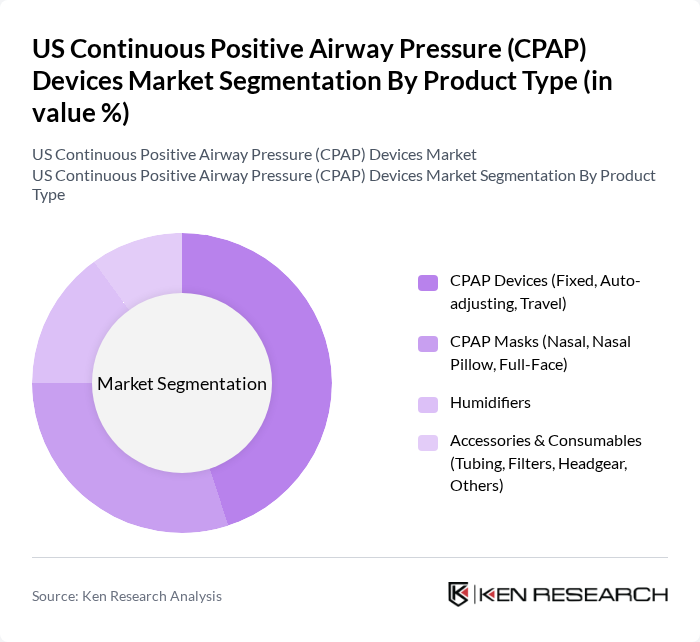
By Product Type:The product type segmentation includes various categories such as CPAP Devices, CPAP Masks, Humidifiers, and Accessories & Consumables. Among these, CPAP Devices, which include fixed, auto-adjusting, and travel variants, dominate the market due to their essential role in treating obstructive sleep apnea, with PAP devices (CPAP, BiPAP, and Auto CPAP) recognized as the gold?standard therapy. The increasing adoption of auto-adjusting and cloud?connected devices, which provide personalized therapy and enable remote monitoring, is particularly notable as they cater to diverse patient needs and support payer requirements for adherence tracking.
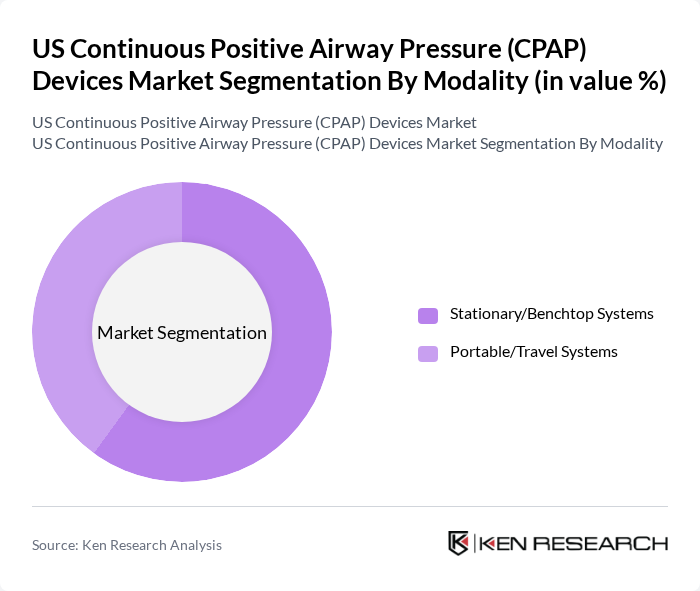
By Modality:The modality segmentation includes Stationary/Benchtop Systems and Portable/Travel Systems. Stationary systems dominate the market due to their widespread use in home care and institutional settings, where they provide consistent and effective therapy and are often integrated with remote monitoring platforms for long?term management of obstructive sleep apnea. The growing trend towards portable systems is also notable, supported by increasing travel, patient preference for mobility, and design improvements that reduce size and noise while maintaining therapeutic performance.
The US Continuous Positive Airway Pressure (CPAP) Devices Market is characterized by a dynamic mix of regional and international players. Leading participants such as ResMed Inc., Koninklijke Philips N.V. (Philips Respironics), Fisher & Paykel Healthcare Corporation Limited, Drive DeVilbiss Healthcare (Drive Medical), 3B Medical, Inc., Somnetics International, Inc., Apex Medical Corp., BMC Medical Co., Ltd. (BMC Medical), Inspire Medical Systems, Inc., Itamar Medical Ltd. (a Zoll Medical company), React Health (formerly 3B Medical/AGS Health, including Z2 CPAP line), Vyaire Medical, Inc., Natus Medical Incorporated, Sleepnet Corporation, Owens & Minor, Inc. (Apria Healthcare) contribute to innovation, geographic expansion, and service delivery in this space.
The future of the CPAP devices market in the United States appears promising, driven by ongoing technological innovations and a growing emphasis on patient-centric care. As telehealth services expand, more patients will have access to remote consultations and follow-up care, enhancing treatment adherence. Additionally, the integration of artificial intelligence in CPAP technology is expected to improve device customization and user experience, ultimately leading to better health outcomes for patients suffering from sleep apnea.
| Segment | Sub-Segments |
|---|---|
| By Product Type | CPAP Devices (Fixed, Auto-adjusting, Travel) CPAP Masks (Nasal, Nasal Pillow, Full-Face) Humidifiers Accessories & Consumables (Tubing, Filters, Headgear, Others) |
| By Modality | Stationary/Benchtop Systems Portable/Travel Systems |
| By Indication | Obstructive Sleep Apnea (OSA) Central Sleep Apnea Complex/Mixed Sleep Apnea Other Respiratory Disorders (e.g., COPD, Post?surgical, Hypoxemia) |
| By End-User | Home Care Settings Hospitals Sleep Clinics & Sleep Labs Ambulatory Surgical Centers & Others |
| By Distribution Channel | Durable Medical Equipment (DME) Providers Hospital & Clinic-based Procurement Online Channels (Manufacturer & E?commerce Platforms) Retail Pharmacies & Others |
| By Region | Northeast Midwest South West |
| By Patient Type | Adults Pediatric Geriatric |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Healthcare Providers | 120 | Pulmonologists, Sleep Medicine Specialists |
| CPAP Device Manufacturers | 90 | Product Managers, Sales Directors |
| Patients Using CPAP Devices | 140 | Current Users, Recent Users |
| Insurance Providers | 80 | Claims Analysts, Policy Underwriters |
| Healthcare Policy Experts | 50 | Health Economists, Regulatory Affairs Specialists |
The US Continuous Positive Airway Pressure (CPAP) Devices Market is valued at approximately USD 4.2 billion, driven by the rising prevalence of obstructive sleep apnea, increased awareness of sleep disorders, and technological advancements in CPAP devices.