Region:Global
Author(s):Rebecca
Product Code:KRAA2421
Pages:81
Published On:August 2025
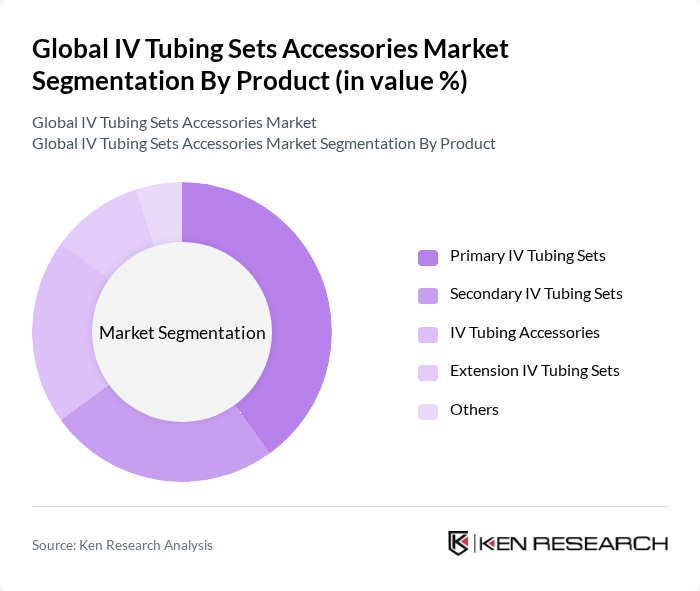
By Product:The product segmentation includes various types of IV tubing sets and accessories that cater to different medical needs. Primary IV tubing sets are widely used in hospitals for fluid administration, while secondary IV tubing sets are essential for administering multiple medications. IV tubing accessories enhance the functionality and safety of these systems, and extension IV tubing sets provide additional length for patient comfort. Other products in this category may include specialized connectors, filters, and microbore tubing designed for precise fluid delivery .
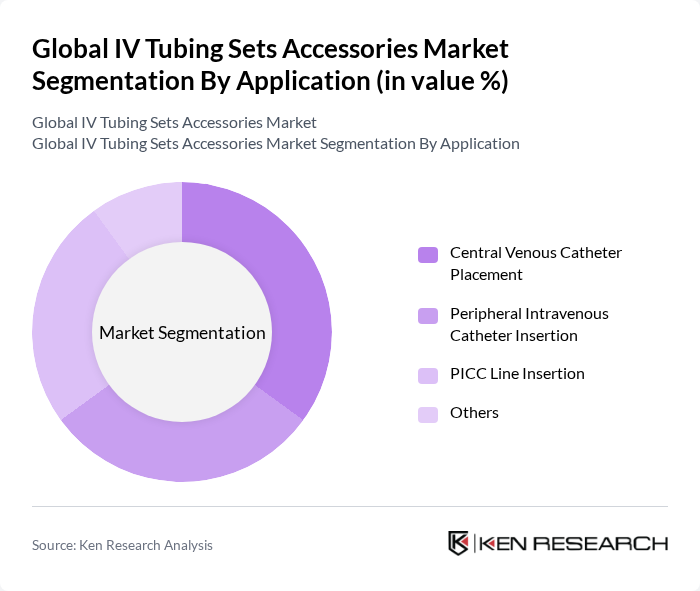
By Application:The application segmentation highlights the various medical procedures that utilize IV tubing sets and accessories. Central venous catheter placement is a critical application, especially in intensive care and oncology settings, while peripheral intravenous catheter insertion is commonly performed in both outpatient and inpatient care. PICC line insertion is gaining traction due to its suitability for long-term therapy, particularly in patients requiring extended medication or nutrition administration. Other applications include blood transfusions, hydration therapies, and parenteral nutrition .
The Global IV Tubing Sets Accessories Market is characterized by a dynamic mix of regional and international players. Leading participants such as Baxter International Inc., B. Braun Melsungen AG, Medtronic plc, Smiths Medical (now part of ICU Medical, Inc.), Terumo Corporation, Fresenius Kabi AG, ICU Medical, Inc., Avanos Medical, Inc. (formerly Halyard Health, Inc.), 3M Company, Johnson & Johnson, ConvaTec Group PLC, Cardinal Health, Inc., Nipro Corporation, Cook Medical, Vygon S.A.S. contribute to innovation, geographic expansion, and service delivery in this space.
The future of the IV tubing sets accessories market appears promising, driven by ongoing technological advancements and an increasing focus on patient-centered care. As healthcare systems evolve, there is a notable shift towards home healthcare solutions, allowing patients to receive IV therapy in the comfort of their homes. Additionally, the integration of IoT technologies in medical devices is expected to enhance monitoring and safety, further propelling market growth. These trends indicate a dynamic landscape for IV accessories in the coming years.
| Segment | Sub-Segments |
|---|---|
| By Product | Primary IV Tubing Sets Secondary IV Tubing Sets IV Tubing Accessories Extension IV Tubing Sets Others |
| By Application | Central Venous Catheter Placement Peripheral Intravenous Catheter Insertion PICC Line Insertion Others |
| By End-User | Hospitals Ambulatory Surgical Centers Home Healthcare Others |
| By Geography | North America Europe Asia-Pacific Middle East & Africa South America Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Hospital Procurement Departments | 80 | Procurement Managers, Supply Chain Coordinators |
| IV Therapy Specialists | 60 | Clinical Nurses, IV Therapy Coordinators |
| Manufacturers of IV Accessories | 40 | Product Managers, R&D Directors |
| Healthcare Facility Administrators | 50 | Facility Managers, Operations Directors |
| Regulatory Bodies and Health Authorities | 40 | Regulatory Affairs Specialists, Compliance Officers |
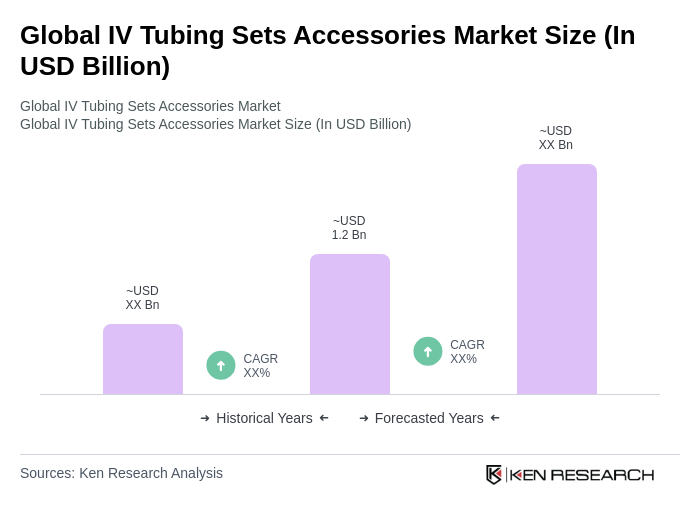
The Global IV Tubing Sets Accessories Market is valued at approximately USD 1.2 billion, reflecting a significant growth driven by the increasing prevalence of chronic diseases and advancements in healthcare infrastructure.