Region:Middle East
Author(s):Shubham
Product Code:KRAD6575
Pages:88
Published On:December 2025
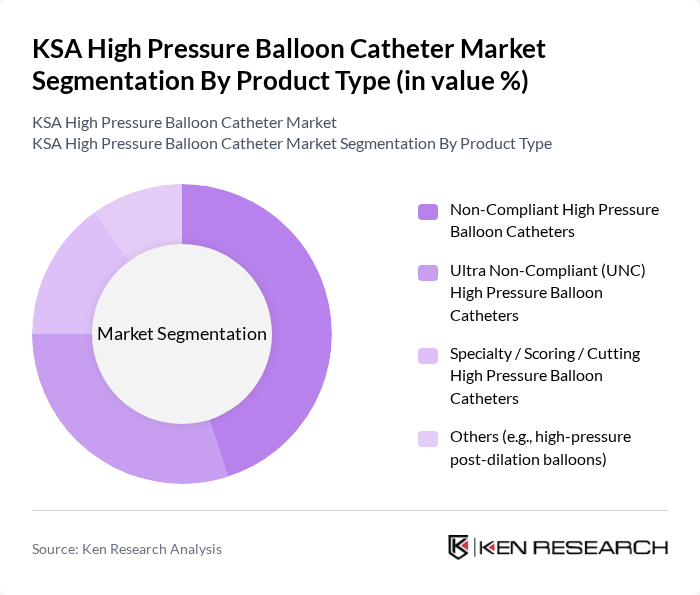
By Product Type:The product type segmentation includes Non-Compliant High Pressure Balloon Catheters, Ultra Non-Compliant (UNC) High Pressure Balloon Catheters, Specialty / Scoring / Cutting High Pressure Balloon Catheters, and Others (e.g., high-pressure post-dilation balloons). Among these, Non-Compliant High Pressure Balloon Catheters are leading the market due to their widespread use in various interventional procedures. Their ability to withstand high pressures without deformation makes them a preferred choice for cardiologists. The increasing number of coronary interventions and the growing preference for minimally invasive techniques further bolster the demand for this sub-segment.
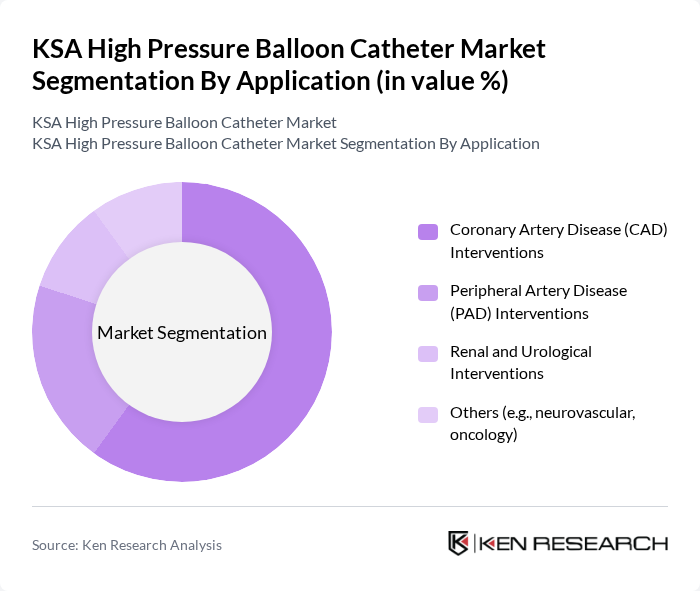
By Application:The application segmentation includes Coronary Artery Disease (CAD) Interventions, Peripheral Artery Disease (PAD) Interventions, Renal and Urological Interventions, and Others (e.g., neurovascular, oncology). The Coronary Artery Disease (CAD) Interventions segment dominates the market, driven by the high prevalence of coronary artery diseases in the region. The increasing number of angioplasty procedures and the growing awareness of cardiovascular health are key factors contributing to the growth of this segment. Additionally, advancements in catheter technology and improved patient outcomes further enhance the adoption of high-pressure balloon catheters in CAD interventions.
The KSA High Pressure Balloon Catheter Market is characterized by a dynamic mix of regional and international players. Leading participants such as Boston Scientific Corporation, Medtronic plc, Abbott Laboratories, Cordis Corporation, B. Braun Melsungen AG, Terumo Corporation, Cook Medical LLC, Biotronik SE & Co. KG, Koninklijke Philips N.V. (Philips Image-Guided Therapy), Teleflex Incorporated, Merit Medical Systems, Inc., BD (Becton, Dickinson and Company), Alvimedica Medical Technologies Inc., MicroPort Scientific Corporation, Local & Regional Distributors in KSA (e.g., Tamer Group, Cigalah Group) contribute to innovation, geographic expansion, and service delivery in this space.
The future of the KSA high-pressure balloon catheter market appears promising, driven by ongoing technological advancements and a growing emphasis on minimally invasive procedures. As healthcare providers increasingly adopt outpatient treatment models, the demand for efficient and effective catheter solutions is expected to rise. Additionally, the integration of digital health technologies will enhance patient monitoring and outcomes, further propelling market growth. The focus on patient-centric care will also shape the development of innovative catheter designs tailored to individual needs.
| Segment | Sub-Segments |
|---|---|
| By Product Type | Non-Compliant High Pressure Balloon Catheters Ultra Non-Compliant (UNC) High Pressure Balloon Catheters Specialty / Scoring / Cutting High Pressure Balloon Catheters Others (e.g., high-pressure post-dilation balloons) |
| By Application | Coronary Artery Disease (CAD) Interventions Peripheral Artery Disease (PAD) Interventions Renal and Urological Interventions Others (e.g., neurovascular, oncology) |
| By Material | Nylon (Polyamide) Polyester (PET) Polyethylene and Co?polymer Blends Others |
| By End-User | Tertiary Care Hospitals & Cardiac Centers Ambulatory Surgical Centers Specialized Cardiology & Vascular Clinics Others |
| By Region | Central Region (incl. Riyadh) Eastern Region (incl. Dammam, Khobar) Western Region (incl. Jeddah, Makkah, Madinah) Southern & Northern Regions |
| By Distribution Channel | Direct Sales to Hospitals & Government Tenders Local Distributors / Importers Group Purchasing Organizations (GPOs) Others |
| By Pricing Tier | Premium High-Performance Catheters Mid-Range Catheters Value / Economy Catheters Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Cardiology Departments in Hospitals | 90 | Cardiologists, Interventional Radiologists |
| Medical Device Distributors | 75 | Sales Managers, Product Specialists |
| Healthcare Procurement Officers | 65 | Procurement Managers, Supply Chain Directors |
| Clinical Research Organizations | 55 | Clinical Researchers, Regulatory Affairs Specialists |
| Patient Advocacy Groups | 45 | Patient Representatives, Healthcare Advocates |
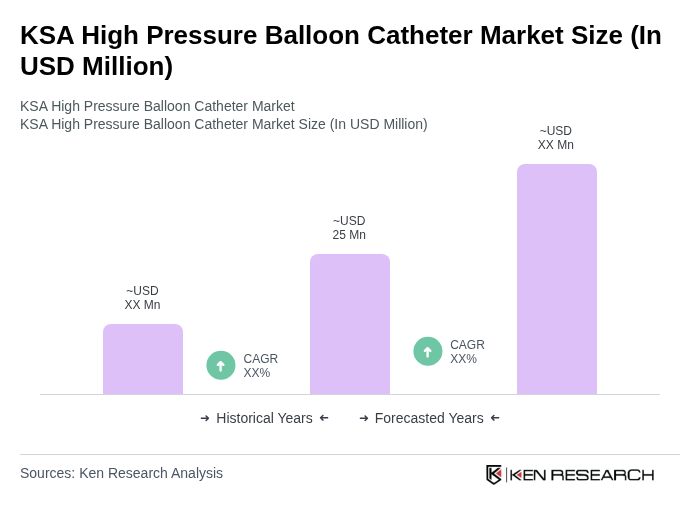
The KSA High Pressure Balloon Catheter Market is valued at approximately USD 25 million, reflecting a five-year historical analysis. This growth is driven by the rising prevalence of cardiovascular diseases and advancements in catheter technology.