Region:Middle East
Author(s):Shubham
Product Code:KRAA8836
Pages:83
Published On:November 2025
By Product Type:
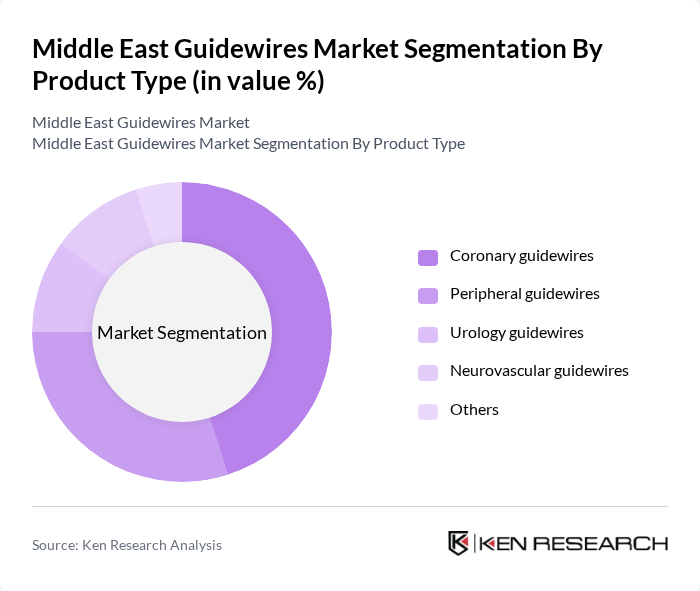
The product type segmentation includes coronary guidewires, peripheral guidewires, urology guidewires, neurovascular guidewires, and others. Among these, coronary guidewires dominate the market due to the high prevalence of coronary artery diseases and the increasing number of angioplasty procedures. The demand for these guidewires is driven by advancements in technology, which enhance their performance and safety during cardiovascular interventions. Peripheral guidewires also show significant growth, attributed to the rising incidence of peripheral vascular diseases. The neurovascular segment is expanding due to increased adoption of endovascular procedures for stroke and aneurysm treatments .
By Coating:
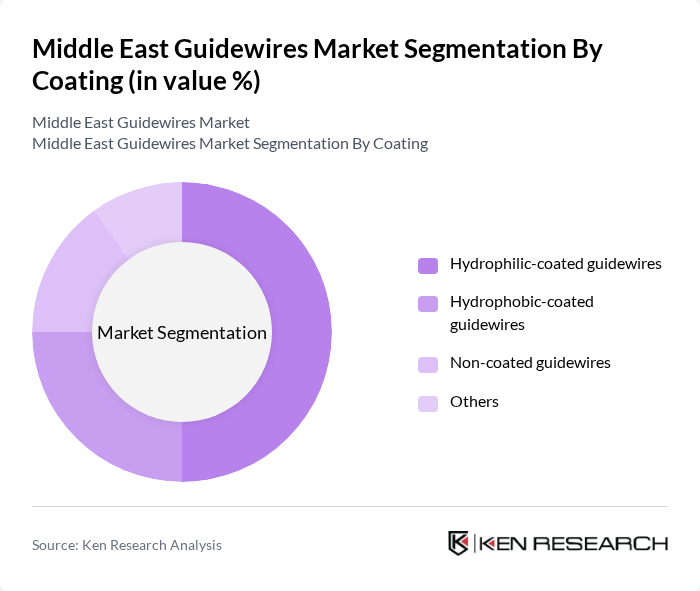
The coating segmentation includes hydrophilic-coated guidewires, hydrophobic-coated guidewires, non-coated guidewires, and others. Hydrophilic-coated guidewires are leading the market due to their enhanced lubricity, which facilitates smoother navigation through blood vessels. This feature is particularly important in complex procedures, making them a preferred choice among healthcare professionals. Hydrophobic-coated guidewires are also gaining traction, especially in specific applications where reduced friction is essential. The trend toward coated guidewires is driven by the need for improved procedural success and reduced complications .
The Middle East Guidewires Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic plc, Boston Scientific Corporation, Abbott Laboratories, Terumo Corporation, Johnson & Johnson (Biosense Webster & Cordis), Cook Medical LLC, B. Braun Melsungen AG, Cardinal Health, Inc., Asahi Intecc Co., Ltd., Merit Medical Systems, Inc., CONMED Corporation, Stryker Corporation, Olympus Corporation, Koninklijke Philips N.V. (Philips Healthcare), Avanos Medical, Inc. (formerly Halyard Health) contribute to innovation, geographic expansion, and service delivery in this space.
The Middle East guidewires market is poised for significant growth, driven by technological advancements and an increasing focus on patient-centric solutions. As healthcare infrastructure expands, particularly in countries like Saudi Arabia and the UAE, the demand for innovative medical devices will rise. Additionally, the integration of digital technologies in healthcare practices will enhance the efficiency of guidewire systems, leading to improved patient outcomes. The market is expected to adapt to these trends, fostering a competitive landscape that prioritizes quality and accessibility.
| Segment | Sub-Segments |
|---|---|
| By Product Type | Coronary guidewires Peripheral guidewires Urology guidewires Neurovascular guidewires Others |
| By Coating | Hydrophilic-coated guidewires Hydrophobic-coated guidewires Non-coated guidewires Others |
| By Material | Stainless steel Nitinol Polymer Others |
| By Application | Cardiovascular procedures Neurological procedures Gastroenterological procedures Urological procedures Others |
| By End-User | Hospitals Ambulatory surgical centers Specialty clinics Diagnostic centers Others |
| By Distribution Channel | Direct sales Distributors Online sales Others |
| By Region | GCC Countries (Saudi Arabia, UAE, Qatar, Kuwait, Oman, Bahrain) Levant Region (Jordan, Lebanon, Syria, Palestine, Iraq) North Africa (Egypt, Morocco, Algeria, Tunisia, Libya) Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Cardiovascular Guidewires | 100 | Interventional Cardiologists, Cardiac Surgeons |
| Neurovascular Guidewires | 80 | Neurosurgeons, Interventional Radiologists |
| Urological Guidewires | 60 | Urologists, Surgical Nurses |
| Gastrointestinal Guidewires | 50 | Gastroenterologists, Endoscopy Technicians |
| General Surgical Guidewires | 70 | General Surgeons, Operating Room Managers |
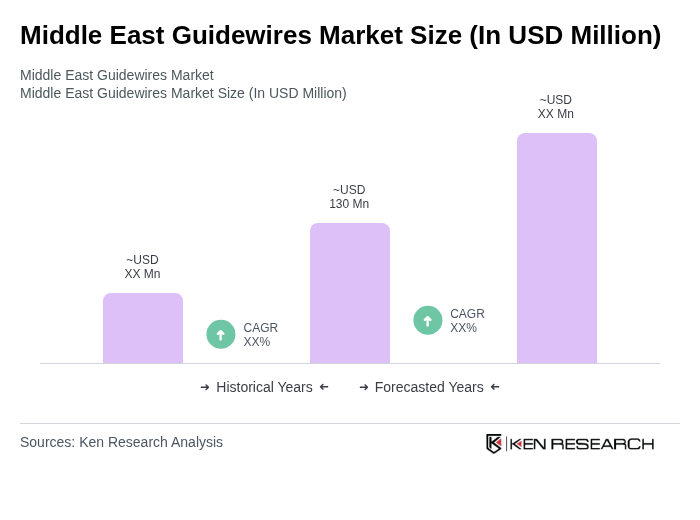
The Middle East Guidewires Market is valued at approximately USD 130 million, driven by factors such as the rising prevalence of cardiovascular diseases, advancements in medical technology, and an increase in minimally invasive surgeries across the region.