Region:Middle East
Author(s):Shubham
Product Code:KRAD5417
Pages:96
Published On:December 2025
By Service Type:The service type segmentation includes various offerings that cater to the specific needs of clients in the plasmid DNA manufacturing sector. The subsegments include Process Development & Optimization, GMP Plasmid DNA Manufacturing, Non-GMP / Research-Grade Plasmid DNA Manufacturing, Analytical Testing & Quality Control Services, and Fill-Finish & Packaging Services. Each of these services plays a crucial role in the overall production process, ensuring that the plasmid DNA meets the required standards for therapeutic applications.
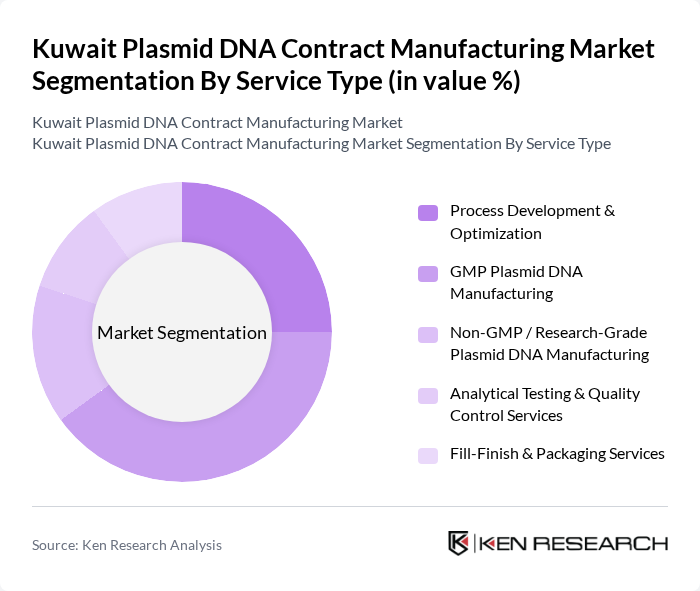
The GMP Plasmid DNA Manufacturing subsegment is currently dominating the market due to the increasing regulatory requirements for the production of therapeutic products. This segment is essential for ensuring that plasmid DNA is produced under stringent quality standards, which is critical for clinical applications. The demand for GMP-grade plasmid DNA is driven by the growing number of clinical trials and the need for high-quality materials in gene therapy and vaccine development. As a result, companies are increasingly investing in GMP facilities to meet these demands.
By Plasmid Grade:The plasmid grade segmentation categorizes the market based on the quality and intended use of the plasmid DNA produced. The subsegments include Research-Grade Plasmid DNA, GMP-Grade Plasmid DNA, High-Supercoiled / High-Concentration Plasmid DNA, and Others. Each grade serves different purposes, with GMP-grade being crucial for clinical applications and research-grade primarily used in laboratory settings.
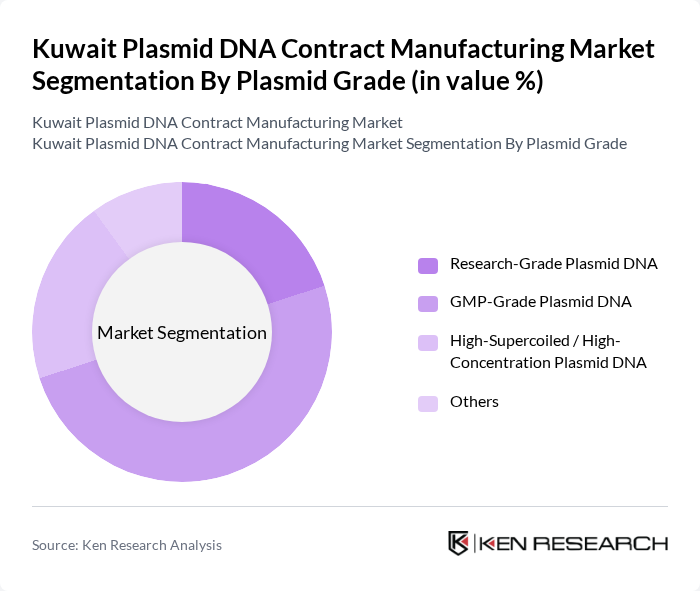
The GMP-Grade Plasmid DNA subsegment is leading the market due to its critical role in the development of therapeutic products. The increasing number of gene therapy and vaccine projects necessitates the use of GMP-grade plasmid DNA, which complies with regulatory standards for safety and efficacy. This trend is further supported by the growing investment in biopharmaceutical research and the need for high-quality materials in clinical applications.
The Kuwait Plasmid DNA Contract Manufacturing Market is characterized by a dynamic mix of regional and international players. Leading participants such as GenScript ProBio, Aldevron LLC, Lonza Group Ltd., WuXi Advanced Therapies (WuXi AppTec Group), Catalent Pharma Solutions, Charles River Laboratories International, Inc., Thermo Fisher Scientific Inc. (including Patheon), Merck KGaA (MilliporeSigma), AGC Biologics, Fujifilm Diosynth Biotechnologies, Bavarian Nordic A/S, SaudiVax, Gulf Biotech Company K.S.C., Kuwait University – Health Sciences Center (Research & Clinical Collaborations), Dasman Diabetes Institute (Translational Research Partner) contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Kuwait plasmid DNA contract manufacturing market appears promising, driven by increasing investments in biopharmaceuticals and advancements in production technologies. As the demand for gene therapies continues to rise, local manufacturers are expected to enhance their capabilities through automation and strategic partnerships. Additionally, the government's supportive policies and funding initiatives will likely create a favorable environment for innovation, positioning Kuwait as a competitive player in the global plasmid DNA landscape.
| Segment | Sub-Segments |
|---|---|
| By Service Type | Process Development & Optimization GMP Plasmid DNA Manufacturing Non-GMP / Research-Grade Plasmid DNA Manufacturing Analytical Testing & Quality Control Services Fill-Finish & Packaging Services |
| By Plasmid Grade | Research-Grade Plasmid DNA GMP-Grade Plasmid DNA High-Supercoiled / High-Concentration Plasmid DNA Others |
| By Application | Gene Therapy & Cell Therapy (CAR-T, TCR, etc.) DNA & RNA Vaccine Development Viral Vector Production (AAV, Lentiviral, Adenoviral) CRISPR / Genome Editing & Synthetic Biology Preclinical & Clinical Research Others |
| By Therapeutic Area | Oncology Infectious Diseases Rare & Genetic Disorders Cardiovascular & Metabolic Diseases Others |
| By Production Scale | Preclinical / Laboratory Scale (milligram level) Clinical Scale (gram to multi-gram level) Commercial Scale (kilogram level) Others |
| By Vector Design | Viral Vector Plasmids (AAV, Lentiviral, Adenoviral backbones) DNA Vaccine Plasmids Gene Expression Plasmids Customized / Synthetic Plasmids Others |
| By Client Type | Multinational Biopharmaceutical & Vaccine Companies Regional Biotechnology & Start-up Firms Academic & Research Institutes in Kuwait Government & Public Health Agencies Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Gene Therapy Applications | 100 | Biotech Researchers, Clinical Trial Managers |
| Vaccine Development | 80 | Pharmaceutical Scientists, Regulatory Affairs Specialists |
| Research Institutions | 60 | Academic Researchers, Lab Managers |
| Contract Manufacturing Services | 70 | Business Development Managers, Operations Directors |
| Quality Control and Assurance | 50 | Quality Assurance Managers, Compliance Officers |
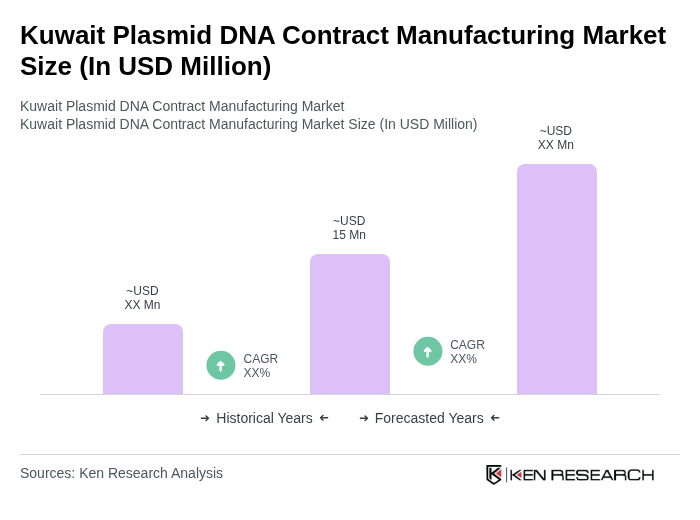
The Kuwait Plasmid DNA Contract Manufacturing Market is valued at approximately USD 15 million, reflecting a five-year historical analysis. This growth is driven by the increasing demand for plasmid DNA in gene therapy and vaccine development.