Region:Middle East
Author(s):Shubham
Product Code:KRAD5547
Pages:83
Published On:December 2025
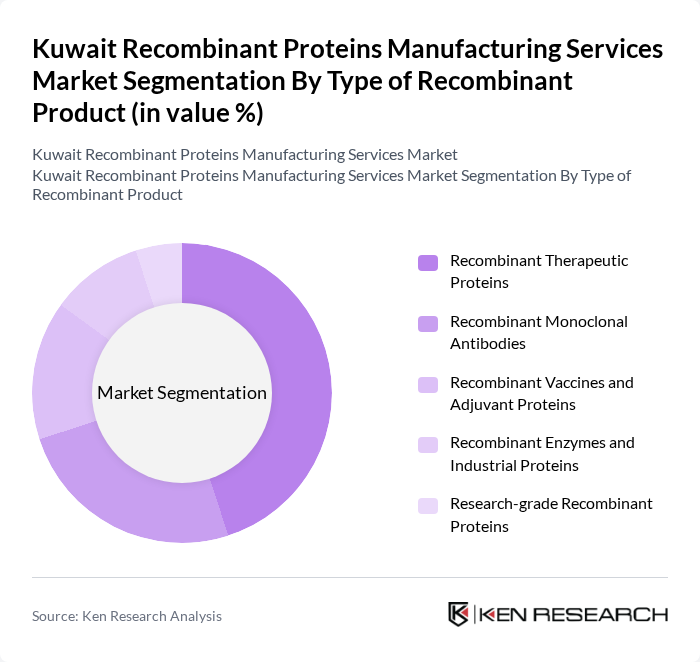
By Type of Recombinant Product:The market is segmented into various types of recombinant products, including therapeutic proteins, monoclonal antibodies, vaccines, enzymes, and research-grade proteins. Among these, recombinant therapeutic proteins are leading the market due to their extensive application in treating chronic diseases and their growing acceptance in clinical settings. The increasing focus on personalized medicine and biologics is driving the demand for these products.
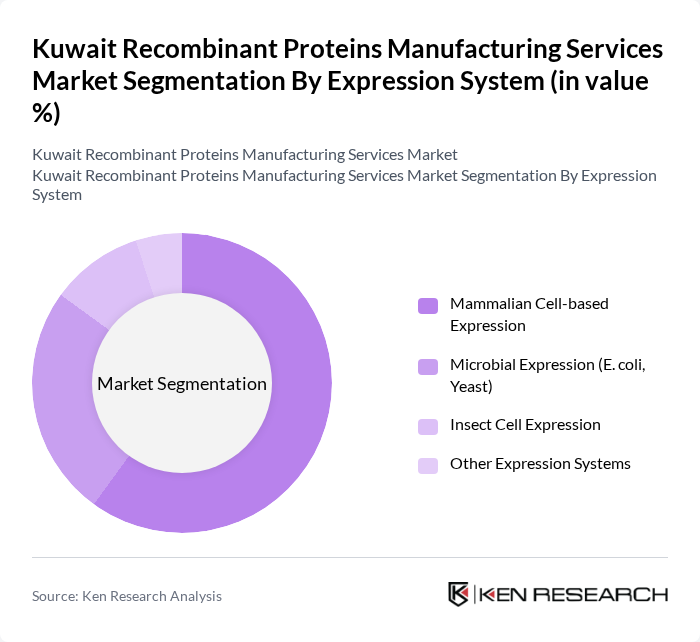
By Expression System:The expression systems used in recombinant protein manufacturing include mammalian cell-based, microbial, insect cell, and other systems. The mammalian cell-based expression system is currently the most dominant due to its ability to produce complex proteins that require post-translational modifications, which are essential for therapeutic efficacy. This trend is supported by the increasing demand for high-quality biologics in the pharmaceutical industry.
The Kuwait Recombinant Proteins Manufacturing Services Market is characterized by a dynamic mix of regional and international players. Leading participants such as Kuwait Institute for Scientific Research (KISR), Dasman Diabetes Institute, Kuwait University – Faculty of Science (Biological Sciences Department), Kuwait University – Faculty of Medicine, Jaber Al Ahmad Center for Molecular Imaging and Nuclear Medicine, Ministry of Health – Kuwait, National Guard Health Affairs – Kuwait, Mubarak Al-Kabeer Hospital Research Laboratories, Sabah Hospital Biomedical Research Center, Kuwait Life Sciences Company (KLSC), Agility Public Warehousing Company – Life Sciences & Healthcare Logistics, Gulf Biotech Company (Bahrain), Saudi Bio (Saudi Arabia), Qatar Biotech (Qatar), Global CDMOs Serving Kuwait (e.g., Samsung Biologics, Lonza, Boehringer Ingelheim BioXcellence) contribute to innovation, geographic expansion, and service delivery in this space.
The future of the recombinant proteins manufacturing services market in Kuwait appears promising, driven by increasing investments in biotechnology and supportive government policies. As the demand for biopharmaceuticals continues to rise, local manufacturers are likely to enhance their production capabilities. Furthermore, the integration of advanced technologies, such as artificial intelligence, is expected to streamline operations and improve product quality, positioning Kuwait as a competitive player in the regional biopharmaceutical landscape.
| Segment | Sub-Segments |
|---|---|
| By Type of Recombinant Product | Recombinant Therapeutic Proteins Recombinant Monoclonal Antibodies Recombinant Vaccines and Adjuvant Proteins Recombinant Enzymes and Industrial Proteins Research-grade Recombinant Proteins |
| By Expression System | Mammalian Cell-based Expression Microbial Expression (E. coli, Yeast) Insect Cell Expression Other Expression Systems |
| By Service Type | Process Development and Optimization Upstream Manufacturing (Cell Culture / Fermentation) Downstream Purification and Analytics Fill–Finish and Packaging Services Quality Control, Regulatory, and Validation Services |
| By Production Scale | Preclinical and Early-Stage Clinical Batches Late-Stage Clinical and Pilot-Scale Production Commercial-scale Manufacturing Small-batch and Custom Protein Production |
| By Client Type | Multinational Biopharmaceutical Companies Regional and Local Pharmaceutical Companies Academic and Research Institutions Government and Non-profit Research Bodies Contract Research and Contract Development Organizations |
| By Application Area | Therapeutics and Clinical Use in vitro Diagnostics and Biomarkers Drug Discovery and Screening Basic Research and Omics Studies Other Industrial and Specialty Applications |
| By Location of Manufacturing Facility | Onshore Facilities within Kuwait Nearshore Facilities in GCC Countries Offshore Facilities (Europe, North America, Asia) Hybrid / Multi-site Manufacturing Models |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Therapeutic Protein Manufacturers | 60 | Production Managers, Quality Assurance Officers |
| Diagnostic Reagents Producers | 50 | Research Scientists, Product Development Managers |
| Biotechnology Research Institutions | 45 | Lab Directors, Principal Investigators |
| Regulatory Bodies and Compliance Experts | 40 | Regulatory Affairs Specialists, Compliance Managers |
| Healthcare Providers Utilizing Recombinant Proteins | 70 | Clinical Managers, Procurement Officers |
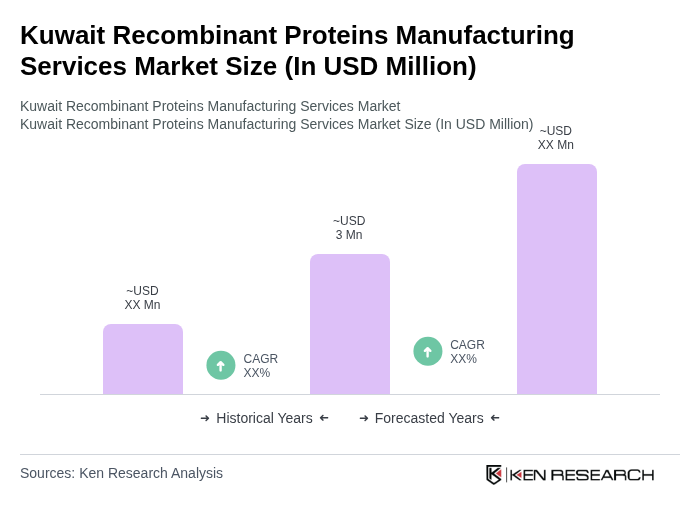
The Kuwait Recombinant Proteins Manufacturing Services Market is valued at approximately USD 3 million, reflecting a five-year historical analysis. This growth is driven by increasing demand for biopharmaceuticals and advancements in biotechnology.