Region:Middle East
Author(s):Geetanshi
Product Code:KRAC3140
Pages:94
Published On:October 2025
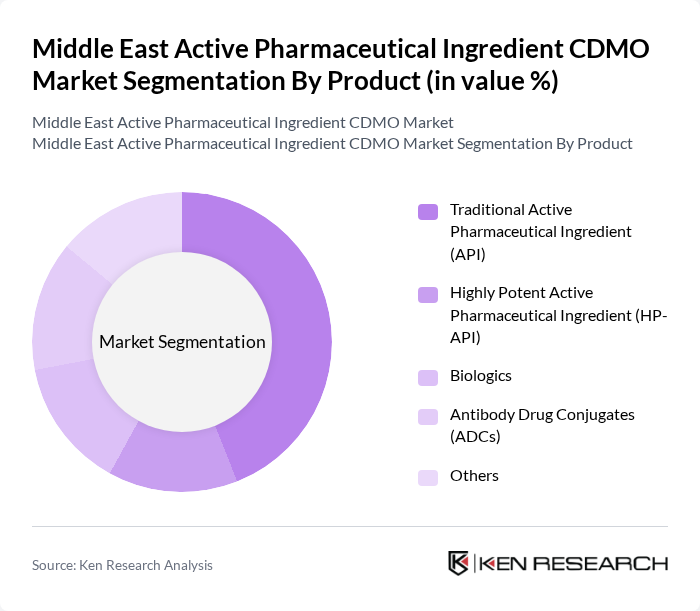
By Product:The product segmentation includes various types of active pharmaceutical ingredients that cater to different therapeutic areas. The subsegments are Traditional Active Pharmaceutical Ingredient (API), Highly Potent Active Pharmaceutical Ingredient (HP-API), Biologics, Antibody Drug Conjugates (ADCs), and Others. Among these, Traditional APIs dominate the market due to their widespread use in generic medications and established manufacturing processes. The demand for HP-APIs is also rising, driven by the increasing prevalence of cancer and other chronic diseases.
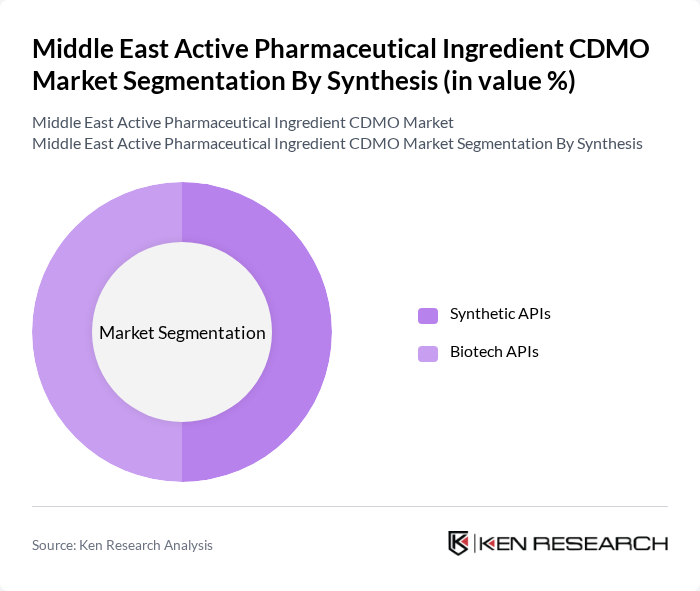
By Synthesis:The synthesis segmentation includes Synthetic APIs and Biotech APIs. Synthetic APIs are the most prevalent in the market due to their cost-effectiveness and established production techniques. Biotech APIs are gaining traction, particularly in the fields of oncology and immunology, as they offer targeted therapies with fewer side effects. The increasing investment in biopharmaceutical research is expected to further boost the growth of Biotech APIs.
The Middle East Active Pharmaceutical Ingredient CDMO Market is characterized by a dynamic mix of regional and international players. Leading participants such as Cambrex Corporation, Recipharm AB, Thermo Fisher Scientific Inc., CordenPharma International, Samsung Biologics, Lonza Group AG, Catalent, Inc., Siegfried Holding AG, Piramal Pharma Solutions, Boehringer Ingelheim International GmbH contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Middle East Active Pharmaceutical Ingredient CDMO market appears promising, driven by increasing healthcare investments and a growing focus on biopharmaceuticals. As local manufacturers enhance their capabilities, the region is likely to see a rise in contract manufacturing services, which will facilitate the production of specialized APIs. Additionally, the emphasis on sustainable practices will encourage CDMOs to adopt eco-friendly technologies, aligning with global trends and enhancing their competitive edge in the market.
| Segment | Sub-Segments |
|---|---|
| By Product | Traditional Active Pharmaceutical Ingredient (API) Highly Potent Active Pharmaceutical Ingredient (HP-API) Biologics Antibody Drug Conjugates (ADCs) Others |
| By Synthesis | Synthetic APIs Biotech APIs |
| By Application | Oncology Cardiovascular Neurology Others |
| By Workflow | Process Development Scale-Up Manufacturing Packaging |
| By Region | GCC Countries Levant Region North Africa Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| API Manufacturing Insights | 50 | Production Managers, Operations Directors |
| Regulatory Compliance in Pharmaceuticals | 60 | Regulatory Affairs Managers, Quality Control Officers |
| Market Trends in CDMO Services | 45 | Business Development Managers, Market Analysts |
| Supply Chain Dynamics for APIs | 55 | Supply Chain Managers, Procurement Specialists |
| Investment Opportunities in API Sector | 40 | Financial Analysts, Venture Capitalists |
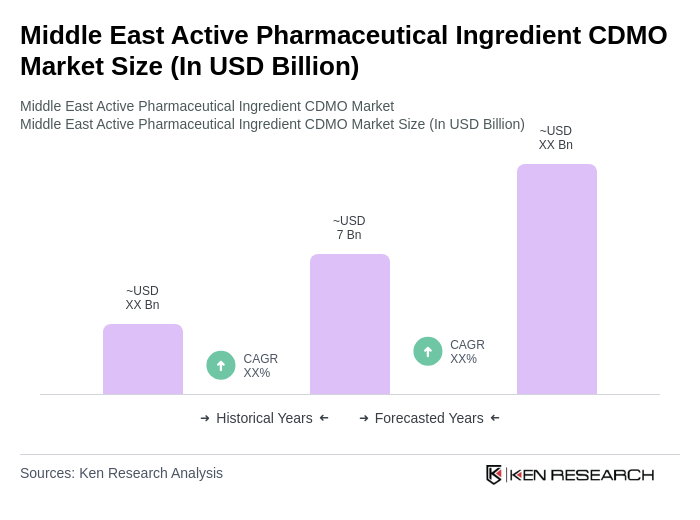
The Middle East Active Pharmaceutical Ingredient CDMO Market is valued at approximately USD 7 billion, reflecting significant growth driven by increasing pharmaceutical demand, chronic disease prevalence, and healthcare infrastructure expansion in the region.