Region:Middle East
Author(s):Shubham
Product Code:KRAA8892
Pages:99
Published On:November 2025
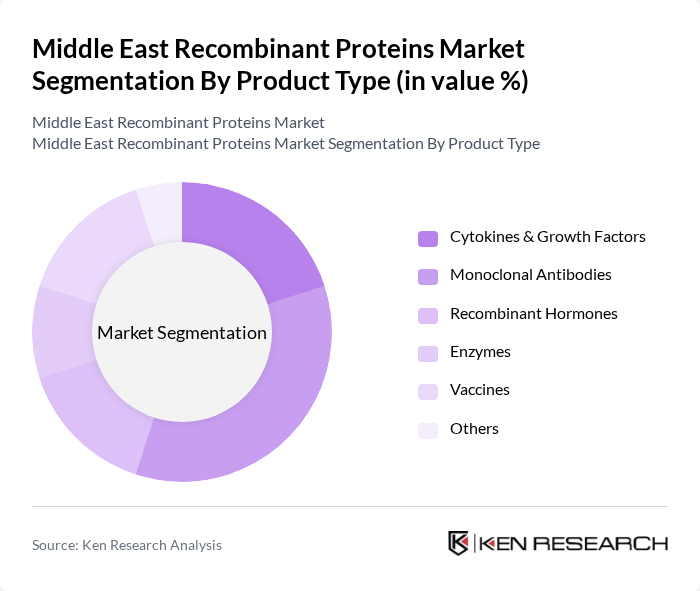
By Product Type:The product type segmentation includes categories such as Cytokines & Growth Factors, Monoclonal Antibodies, Recombinant Hormones, Enzymes, Vaccines, and Others. Among these, Monoclonal Antibodies are leading the market due to their widespread application in cancer treatment and autoimmune diseases. The increasing demand for targeted therapies, immunotherapies, and personalized medicine is driving the growth of this sub-segment .
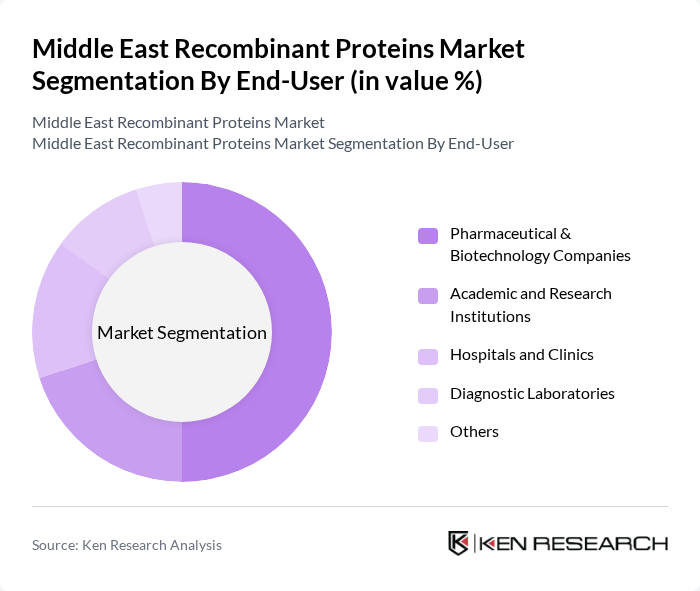
By End-User:The end-user segmentation comprises Pharmaceutical & Biotechnology Companies, Academic and Research Institutions, Hospitals and Clinics, Diagnostic Laboratories, and Others. Pharmaceutical & Biotechnology Companies dominate this segment, driven by their extensive research capabilities, the need for innovative therapies, and growing collaborations with research institutions and healthcare providers to accelerate recombinant protein development .
The Middle East Recombinant Proteins Market is characterized by a dynamic mix of regional and international players. Leading participants such as Amgen Inc., Roche Holding AG, Merck & Co., Inc., Sanofi S.A., Pfizer Inc., AbbVie Inc., GSK plc, Regeneron Pharmaceuticals, Inc., Takeda Pharmaceutical Company Limited, Novartis AG, Bayer AG, Biogen Inc., Eli Lilly and Company, CSL Limited, Lonza Group AG contribute to innovation, geographic expansion, and service delivery in this space.
The future of the recombinant proteins market in the Middle East appears promising, driven by technological advancements and increasing healthcare investments. As countries in the region enhance their healthcare infrastructure, the demand for innovative biopharmaceuticals is expected to rise. Furthermore, collaborations between biotech firms and research institutions are likely to accelerate the development of novel therapies, positioning the region as a competitive player in the global biopharmaceutical landscape.
| Segment | Sub-Segments |
|---|---|
| By Product Type | Cytokines & Growth Factors Monoclonal Antibodies Recombinant Hormones Enzymes Vaccines Others |
| By End-User | Pharmaceutical & Biotechnology Companies Academic and Research Institutions Hospitals and Clinics Diagnostic Laboratories Others |
| By Application | Therapeutics (Autoimmune, Cardiovascular, Oncology) Diagnostics & In Vitro Diagnostics Drug Discovery & Development Basic Research Others |
| By Expression System | Mammalian Cell Culture Microbial Systems (Bacterial & Yeast) Plant-Based Systems Insect Cell Systems Others |
| By Distribution Channel | Direct Sales Distributors & Wholesalers Online Sales Others |
| By Region | GCC Countries (Saudi Arabia, UAE, Kuwait, Qatar, Bahrain, Oman) Levant Region (Egypt, Jordan, Lebanon, Palestine) North Africa (Morocco, Tunisia, Algeria) Others |
| By Regulatory Compliance | FDA Approved EMA Approved WHO Guidelines Compliant Regional Authority Approved Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Biopharmaceutical Companies | 100 | R&D Directors, Product Managers |
| Academic Research Institutions | 60 | Lead Researchers, Lab Managers |
| Regulatory Bodies | 40 | Policy Makers, Compliance Officers |
| Healthcare Providers | 70 | Clinical Directors, Pharmacists |
| Investors in Biotechnology | 50 | Venture Capitalists, Financial Analysts |
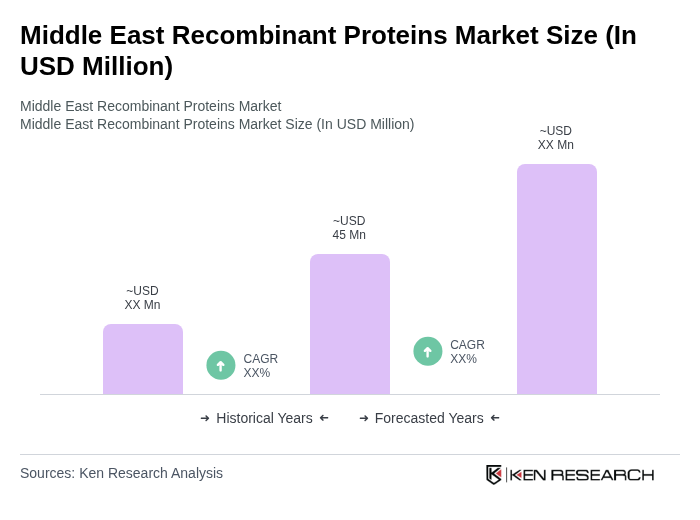
The Middle East Recombinant Proteins Market is valued at approximately USD 45 million, reflecting a five-year historical analysis. This growth is attributed to advancements in biotechnology and increasing demand for therapeutic and diagnostic applications.