Region:Middle East
Author(s):Dev
Product Code:KRAD5295
Pages:99
Published On:December 2025
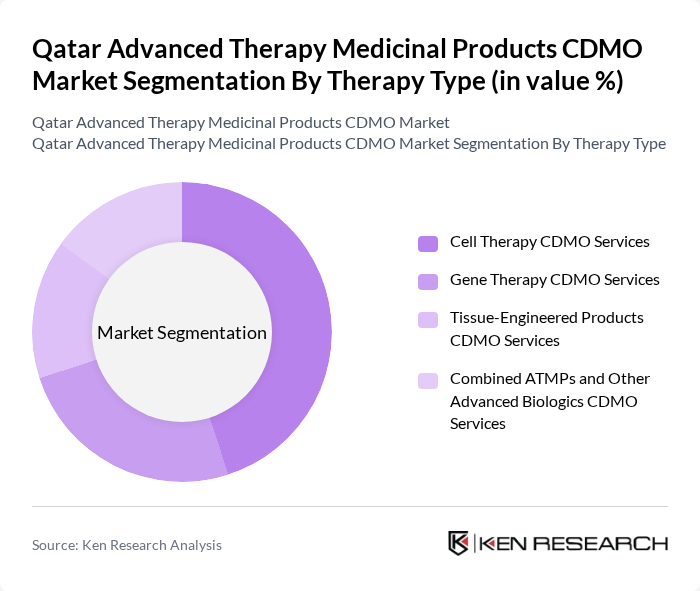
By Therapy Type:The market is segmented into four primary therapy types: Cell Therapy CDMO Services, Gene Therapy CDMO Services, Tissue-Engineered Products CDMO Services, and Combined ATMPs and Other Advanced Biologics CDMO Services. Among these, Cell Therapy CDMO Services is currently the leading segment, driven by the increasing demand for regenerative medicine and the growing number of clinical trials focusing on cell-based therapies. The rise in chronic diseases and the need for personalized treatment options further bolster this segment's growth.
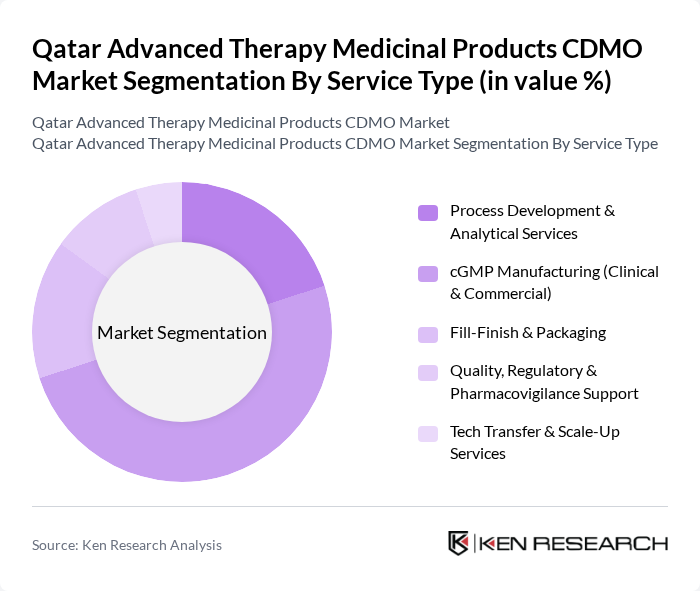
By Service Type:The service type segmentation includes Process Development & Analytical Services, cGMP Manufacturing (Clinical & Commercial), Fill-Finish & Packaging, Quality, Regulatory & Pharmacovigilance Support, and Tech Transfer & Scale-Up Services. The cGMP Manufacturing segment is the most significant contributor to the market, as it ensures compliance with regulatory standards and is essential for the production of safe and effective advanced therapies. The increasing number of biopharmaceutical companies seeking cGMP services to meet regulatory requirements is driving this segment's growth.
The Qatar Advanced Therapy Medicinal Products CDMO Market is characterized by a dynamic mix of regional and international players. Leading participants such as Qatar Biotech, Qatar Advanced Pharmaceutical Industries, Sidra Medicine – Cell Therapy & Regenerative Medicine Program, Hamad Medical Corporation – Translational Research & Cell Therapy Units, Qatar Biomedical Research Institute (QBRI), Qatar Pharma, Gulf Pharmaceutical Industries (Julphar) – Regional CDMO Operations, Lonza Group Ltd – Cell & Gene Therapy CDMO Division, Catalent Inc. – Advanced Therapies CDMO, Thermo Fisher Scientific Inc. – Patheon Pharma Services, Cytiva, WuXi AppTec Co. Ltd – Advanced Therapies CDMO, Charles River Laboratories International Inc., AGC Biologics, Samsung Biologics – Cell & Gene Therapy Services contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Qatar Advanced Therapy Medicinal Products CDMO market appears promising, driven by ongoing investments in biotechnology and healthcare infrastructure. As the demand for personalized medicine continues to rise, local companies are expected to enhance their capabilities in gene and cell therapies. Furthermore, collaborations between academic institutions and industry players will likely foster innovation, leading to the development of novel therapies that address unmet medical needs in the region.
| Segment | Sub-Segments |
|---|---|
| By Therapy Type | Cell Therapy CDMO Services Gene Therapy CDMO Services Tissue-Engineered Products CDMO Services Combined ATMPs and Other Advanced Biologics CDMO Services |
| By Service Type | Process Development & Analytical Services cGMP Manufacturing (Clinical & Commercial) Fill-Finish & Packaging Quality, Regulatory & Pharmacovigilance Support Tech Transfer & Scale-Up Services |
| By Development Phase | Pre-clinical Clinical (Phase I–III) Commercial |
| By Sponsoring Client Type | Global Biopharmaceutical Companies Emerging Biotech & Start-ups Academic & Research Institutions Government & Public Healthcare Entities |
| By Manufacturing Modality | Autologous Manufacturing Allogeneic Manufacturing Viral Vector & Plasmid Manufacturing Ancillary Reagents & Support Services |
| By Engagement Model | Project-Based Contracts Long-Term Strategic Partnerships Regional Technology-Transfer & Localization Agreements |
| By Geography (Within Qatar & External Client Base) | Domestic (Qatar-Based Sponsors) Inbound Regional Clients (GCC & Middle East) Global Sponsors Utilizing Qatar Facilities Joint Programs with International Organizations |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Gene Therapy Development | 45 | Research Scientists, Clinical Trial Managers |
| Cell Therapy Manufacturing | 40 | Production Managers, Quality Assurance Officers |
| Tissue Engineering Applications | 40 | Biomedical Engineers, Product Development Leads |
| Regulatory Compliance in ATMPs | 40 | Regulatory Affairs Specialists, Compliance Managers |
| Market Access Strategies for ATMPs | 45 | Market Access Managers, Health Economists |
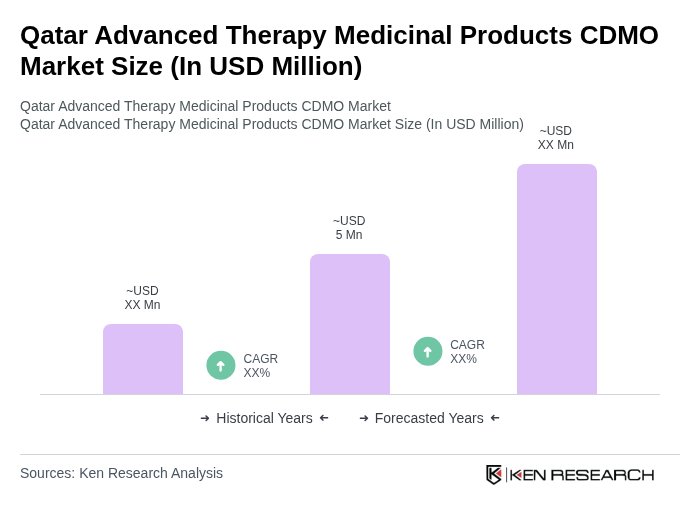
The Qatar Advanced Therapy Medicinal Products CDMO Market is valued at approximately USD 5 million, reflecting a five-year historical analysis. This growth is attributed to increased healthcare investments, chronic disease prevalence, and demand for personalized medicine.