Region:Middle East
Author(s):Geetanshi
Product Code:KRAE1769
Pages:92
Published On:December 2025
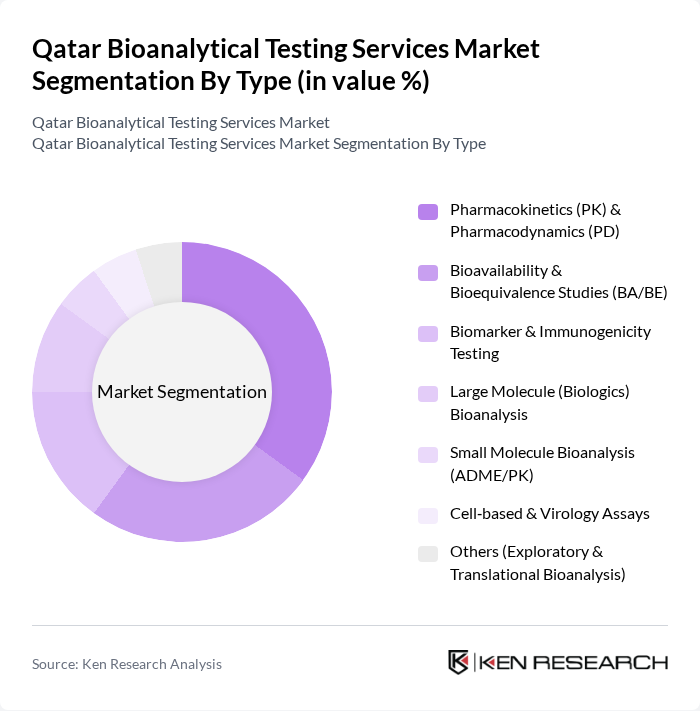
By Type:The bioanalytical testing services market is segmented into various types, including Pharmacokinetics (PK) & Pharmacodynamics (PD), Bioavailability & Bioequivalence Studies (BA/BE), Biomarker & Immunogenicity Testing, Large Molecule (Biologics) Bioanalysis, Small Molecule Bioanalysis (ADME/PK), Cell-based & Virology Assays, and Others (Exploratory & Translational Bioanalysis). Among these, Pharmacokinetics (PK) & Pharmacodynamics (PD) is the leading sub-segment due to its critical role in drug development and regulatory submissions. The increasing focus on personalized medicine and the need for precise dosing regimens further drive the demand for these services.
By End-User:The end-user segmentation includes Pharmaceutical & Biopharmaceutical Companies, Biotechnology & Start-up Ventures, Contract Research Organizations (CROs) & CDMOs, Hospitals, Academic & Research Institutions, Government Agencies & Funding Bodies, and Others. Pharmaceutical & Biopharmaceutical Companies dominate this segment due to their extensive reliance on bioanalytical testing for drug development and regulatory compliance. The increasing number of drug approvals and the growing pipeline of new therapies are key factors driving this demand.

The Qatar Bioanalytical Testing Services Market is characterized by a dynamic mix of regional and international players. Leading participants such asHamad Medical Corporation (HMC) – Central Laboratory & Clinical Research Center,Sidra Medicine – Research & Clinical Laboratory Services,Qatar University – Biomedical Research Center & Health Cluster Labs,Qatar Biomedical Research Institute (QBRI), Hamad Bin Khalifa University,Weill Cornell Medicine – Qatar (WCM?Q) – Clinical Research & Core Labs,Aspetar Orthopaedic and Sports Medicine Hospital – Sports Medicine & Bioanalytical Labs,Sidra Medicine – Genomics & Precision Medicine Core Facilities,Qatar Science & Technology Park (QSTP) – Hosted Life Science & CRO Labs,Qatar Foundation for Education, Science and Community Development – Research Ecosystem Enabler,Qatar National Research Fund (QNRF) – Funding Body Driving Bioanalytical Capacity,Qatar Red Crescent Society – Public Health & Laboratory Collaboration Programs,Al Ahli Hospital – Clinical Laboratory & Diagnostic Services,Al Emadi Hospital – Clinical Laboratory & Diagnostic Services,Doha Clinic Hospital – Clinical Laboratory Services,Mowasalat Medical Center & Other Private Hospital Labs (Aggregated Profile)contribute to innovation, geographic expansion, and service delivery in this space.
The future of the bioanalytical testing services market in Qatar appears promising, driven by advancements in technology and increasing healthcare investments. As personalized medicine gains traction, the demand for tailored testing solutions will rise, necessitating innovative bioanalytical methods. Additionally, collaborations between private firms and academic institutions are expected to enhance research capabilities, fostering a more robust testing environment. These trends will likely position Qatar as a regional hub for bioanalytical services, attracting global partnerships and investments.
| Segment | Sub-Segments |
|---|---|
| By Type | **Pharmacokinetics (PK) & Pharmacodynamics (PD)** **Bioavailability & Bioequivalence Studies (BA/BE)** **Biomarker & Immunogenicity Testing** **Large Molecule (Biologics) Bioanalysis** **Small Molecule Bioanalysis (ADME/PK)** **Cell?based & Virology Assays** **Others (Exploratory & Translational Bioanalysis)** |
| By End-User | **Pharmaceutical & Biopharmaceutical Companies** **Biotechnology & Start?up Ventures** **Contract Research Organizations (CROs) & CDMOs** **Hospitals, Academic & Research Institutions** **Government Agencies & Funding Bodies** **Others** |
| By Application | **Preclinical Studies (GLP & Non?GLP)** **Clinical Trials (Phase I–IV)** **Regulatory Submissions & Post?marketing Surveillance** **Therapeutic Drug Monitoring & Routine Clinical Testing** **Translational & Precision Medicine Research** **Others** |
| By Sample Type | **Plasma/Serum & Whole Blood Samples** **Urine Samples** **Tissue & Biopsy Samples** **Saliva, CSF & Other Body Fluids** **Others (Cell Pellets, Cultured Media, etc.)** |
| By Region | **Doha** **Al Rayyan** **Al Wakrah** **Al Khor & Northern Municipalities** **Others** |
| By Technology | **LC?MS/MS & High?resolution Mass Spectrometry** **HPLC/UPLC & Chromatography Platforms** **Ligand Binding Assays (ELISA, ECL, etc.)** **Flow Cytometry & Cell?based Assay Platforms** **Molecular Assays (qPCR, NGS?based Quantification)** **Others** |
| By Service Type | **Bioanalytical Testing & Method Development/Validation** **Regulatory & Scientific Consulting Services** **Quality Assurance, Auditing & Compliance Support** **Data Management, Bioinformatics & Reporting Services** **Training & Technology Transfer Services** **Others** |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Pharmaceutical Bioanalytical Testing | 100 | Laboratory Managers, R&D Directors |
| Clinical Research Organizations | 90 | Clinical Operations Managers, Regulatory Affairs Specialists |
| Biotechnology Firms | 80 | Product Development Managers, Quality Control Analysts |
| Academic Research Institutions | 70 | Research Scientists, Lab Technicians |
| Government Health Agencies | 60 | Policy Makers, Health Program Coordinators |
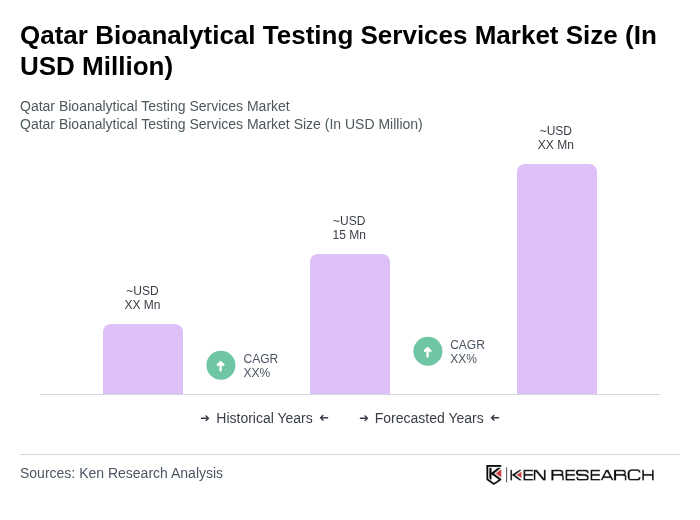
The Qatar Bioanalytical Testing Services Market is valued at approximately USD 15 million, reflecting a five-year historical analysis. This growth is driven by increased demand for advanced testing services in the pharmaceutical and biotechnology sectors.