Region:Middle East
Author(s):Geetanshi
Product Code:KRAC2405
Pages:92
Published On:October 2025
By Type:The market is segmented into various types of validation services, including Process Validation, Cleaning Validation, Equipment Validation, Method Validation, Software Validation, Facility Validation, Extractables & Leachables Testing, Viral Clearance Testing, and Others. Each of these sub-segments plays a crucial role in ensuring the integrity and compliance of bioprocesses. The growing emphasis on regulatory compliance and the expansion of biologics and biosimilars production have intensified the need for comprehensive validation services across all process components.
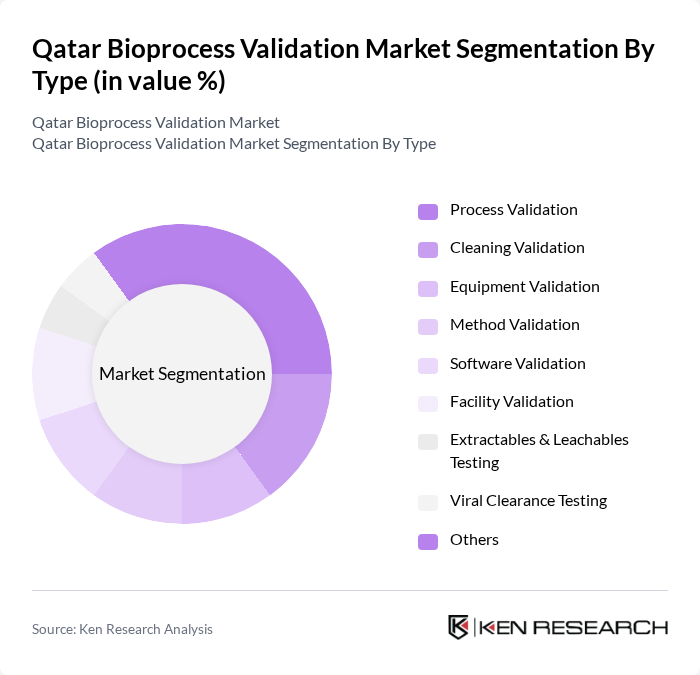
The Process Validation sub-segment is currently dominating the market due to its critical importance in ensuring that biopharmaceutical processes consistently produce products meeting predetermined specifications. This segment is driven by the increasing regulatory scrutiny and the need for compliance with Good Manufacturing Practices (GMP). Regulatory authorities including the FDA and EMA have intensified monitoring of process validation procedures, contributing to increased expenditure on validation services and technologies. The demand for Process Validation is further fueled by the growing biopharmaceutical sector in Qatar, which necessitates rigorous validation protocols to ensure product safety and efficacy.
By End-User:The market is segmented by end-users, including Pharmaceutical Companies, Biotechnology Firms, Contract Research Organizations (CROs), Contract Manufacturing Organizations (CMOs), Academic & Research Institutions, Government Agencies, and Others. Each end-user category has distinct validation needs based on their operational requirements and regulatory obligations. The growth in biosimilars production and the emergence of cell and gene therapy manufacturing have propelled demand for validation services across all end-user segments.
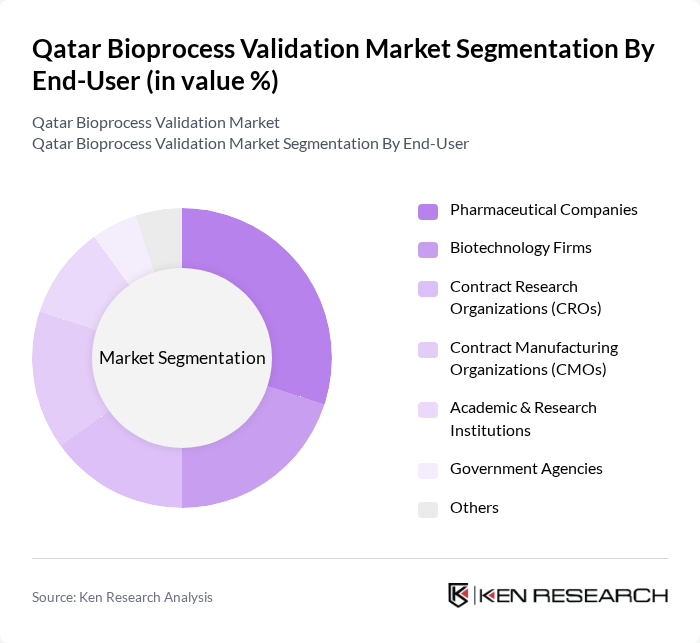
Pharmaceutical Companies are the leading end-users in the market, accounting for a significant share due to their extensive need for validation services to comply with stringent regulatory requirements. The increasing focus on drug safety and efficacy, coupled with the rising number of biopharmaceutical products entering the market, drives the demand for validation services among these companies. The trend toward outsourcing validation processes to specialized Contract Manufacturing Organizations and Contract Research Organizations has accelerated, particularly as these service providers have expanded their capabilities to address demand for advanced therapeutics including mRNA therapies and biosimilars. Additionally, the growing trend of outsourcing validation processes to specialized service providers is further enhancing the market dynamics.
The Qatar Bioprocess Validation Market is characterized by a dynamic mix of regional and international players. Leading participants such as Thermo Fisher Scientific, Merck KGaA, Sartorius AG, Charles River Laboratories, Bio-Rad Laboratories, Eppendorf AG, GE HealthCare, Agilent Technologies, Pall Corporation, Fujifilm Diosynth Biotechnologies, Lonza Group, WuXi AppTec, Catalent, Inc., SGS SA, Eurofins Scientific SE, Cobetter Filtration Equipment Co., Ltd., MEISSNER FILTRATION PRODUCTS, INC., ProBioGen AG, Pacific BioLabs, Nelson Laboratories, LLC contribute to innovation, geographic expansion, and service delivery in this space.
The future of the bioprocess validation market in Qatar appears promising, driven by ongoing investments in biotechnology and a commitment to regulatory compliance. As the demand for biopharmaceuticals continues to rise, companies are likely to adopt more advanced validation techniques, including automation and AI integration. Furthermore, the expansion of biomanufacturing facilities will enhance local production capabilities, positioning Qatar as a key player in the regional biopharmaceutical landscape.
| Segment | Sub-Segments |
|---|---|
| By Type | Process Validation Cleaning Validation Equipment Validation Method Validation Software Validation Facility Validation Extractables & Leachables Testing Viral Clearance Testing Others |
| By End-User | Pharmaceutical Companies Biotechnology Firms Contract Research Organizations (CROs) Contract Manufacturing Organizations (CMOs) Academic & Research Institutions Government Agencies Others |
| By Application | Drug Development Clinical Trials Quality Control Regulatory Compliance Biosimilar & Biologics Production Cell & Gene Therapy Manufacturing Others |
| By Service Type | Consulting Services Testing Services Training Services Documentation Services Digital Validation Solutions Others |
| By Region | Doha Al Rayyan Umm Salal Al Wakrah Others |
| By Compliance Level | Full Compliance Partial Compliance Non-Compliance |
| By Investment Source | Private Investments Government Funding International Grants Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Biopharmaceutical Validation Services | 100 | Quality Assurance Managers, Validation Specialists |
| Regulatory Compliance in Bioprocessing | 60 | Regulatory Affairs Specialists, Compliance Managers |
| Cell and Gene Therapy Validation | 50 | Research Scientists, Process Development Managers |
| Vaccine Production Validation | 70 | Production Managers, Quality Control Analysts |
| Analytical Method Validation | 40 | Laboratory Managers, Analytical Chemists |
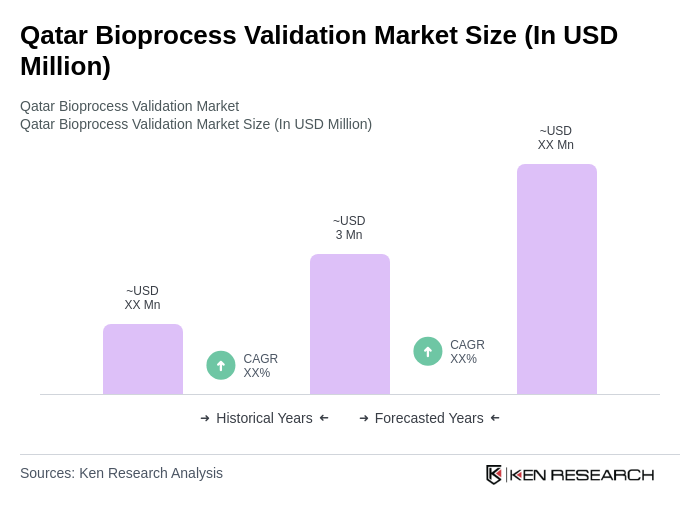
The Qatar Bioprocess Validation Market is valued at approximately USD 3 million, reflecting a five-year historical analysis. This valuation is driven by the increasing demand for biopharmaceuticals and stringent regulatory requirements.