Region:Middle East
Author(s):Dev
Product Code:KRAC8810
Pages:84
Published On:November 2025
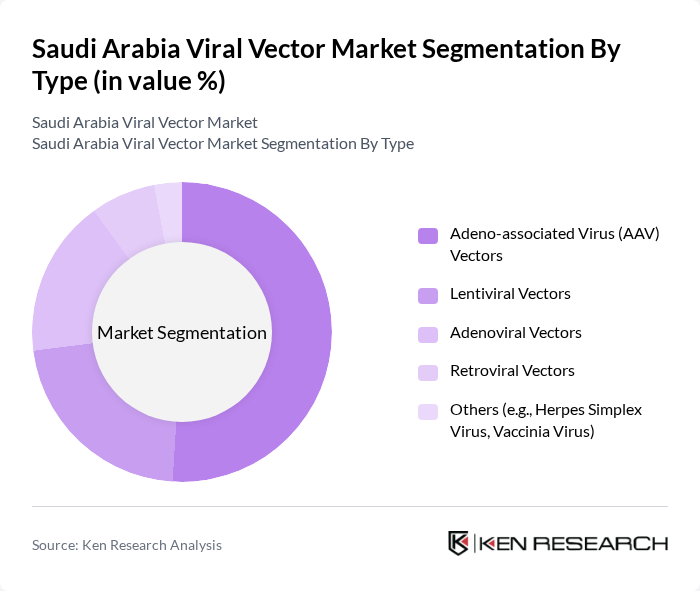
By Type:The market is segmented into various types of viral vectors, including Adeno-associated Virus (AAV) Vectors, Lentiviral Vectors, Adenoviral Vectors, Retroviral Vectors, and Others (e.g., Herpes Simplex Virus, Vaccinia Virus). Among these, Adeno-associated Virus (AAV) Vectors are gaining significant traction due to their favorable safety profile and efficiency in gene delivery, making them a preferred choice for gene therapy applications .
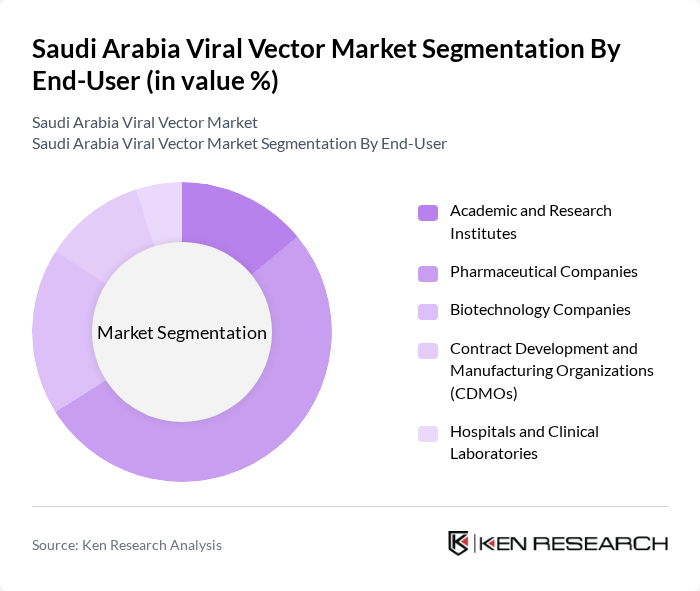
By End-User:The end-user segmentation includes Academic and Research Institutes, Pharmaceutical Companies, Biotechnology Companies, Contract Development and Manufacturing Organizations (CDMOs), Hospitals and Clinical Laboratories, and Others. Pharmaceutical Companies are leading this segment due to their extensive research and development activities in gene therapies and vaccines, which require advanced viral vector technologies .
The Saudi Arabia Viral Vector Market is characterized by a dynamic mix of regional and international players. Leading participants such as Oxford Biomedica, Lonza Group Ltd, Thermo Fisher Scientific Inc., Biogen Inc., Roche Holding AG, REGENXBIO Inc., UniQure N.V., Bayer AG, Sanofi S.A., Batavia Biosciences, Wuxi AppTec Co. Ltd., Pfizer Inc., Astellas Pharma Inc., Genscript Biotech Corporation, Biomarin Pharmaceutical Inc. contribute to innovation, geographic expansion, and service delivery in this space .
The future of the viral vector market in Saudi Arabia appears promising, driven by technological advancements and increasing healthcare investments. The integration of artificial intelligence in vector design is expected to enhance the efficiency of therapeutic development. Additionally, the growing focus on personalized medicine will likely create new avenues for viral vector applications, enabling tailored treatments for patients. As the market evolves, collaboration between public and private sectors will be crucial in overcoming existing challenges and fostering innovation.
| Segment | Sub-Segments |
|---|---|
| By Type | Adeno-associated Virus (AAV) Vectors Lentiviral Vectors Adenoviral Vectors Retroviral Vectors Others (e.g., Herpes Simplex Virus, Vaccinia Virus) |
| By End-User | Academic and Research Institutes Pharmaceutical Companies Biotechnology Companies Contract Development and Manufacturing Organizations (CDMOs) Hospitals and Clinical Laboratories Others |
| By Application | Gene Therapy Vaccine Development Oncology (Oncolytic Virus Therapy) Infectious Disease Research Others |
| By Delivery Method | In Vivo Delivery Ex Vivo Delivery Others |
| By Region | Central Region (Riyadh, Qassim, etc.) Eastern Region (Dammam, Al Khobar, etc.) Western Region (Jeddah, Makkah, Madinah, etc.) Southern Region (Abha, Jizan, etc.) |
| By Research Phase | Preclinical Clinical Trials Commercialization Others |
| By Funding Source | Government Grants Private Investments Venture Capital Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Gene Therapy Developers | 60 | Biotech Researchers, Product Managers |
| Vaccine Manufacturers | 50 | Production Managers, Quality Assurance Leads |
| Academic Research Institutions | 45 | Principal Investigators, Lab Directors |
| Regulatory Bodies | 40 | Regulatory Affairs Specialists, Policy Advisors |
| Healthcare Providers | 55 | Clinical Researchers, Healthcare Administrators |
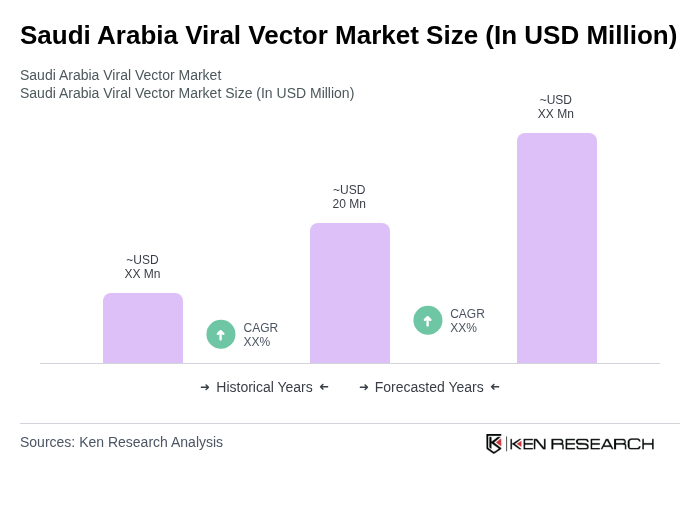
The Saudi Arabia Viral Vector Market is valued at approximately USD 20 million, driven by advancements in gene therapy, vaccine development, and increased investments in biotechnology and pharmaceutical research.