Region:Middle East
Author(s):Dev
Product Code:KRAA8396
Pages:81
Published On:November 2025
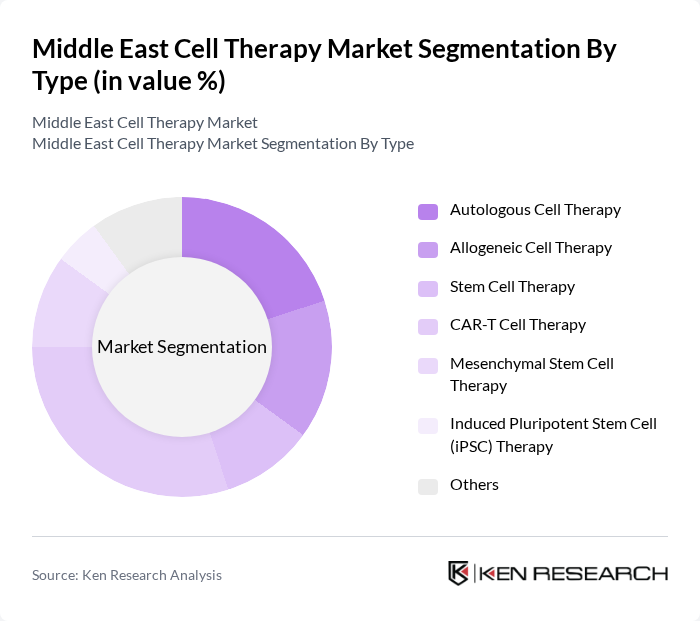
By Type:The market is segmented into various types of cell therapies, including Autologous Cell Therapy, Allogeneic Cell Therapy, Stem Cell Therapy, CAR-T Cell Therapy, Mesenchymal Stem Cell Therapy, Induced Pluripotent Stem Cell (iPSC) Therapy, and Others. Among these, CAR-T Cell Therapy is gaining significant traction due to its effectiveness in treating hematologic malignancies. The increasing number of clinical trials and approvals for CAR-T therapies, particularly in Israel and the UAE, is driving its prominence in the market .
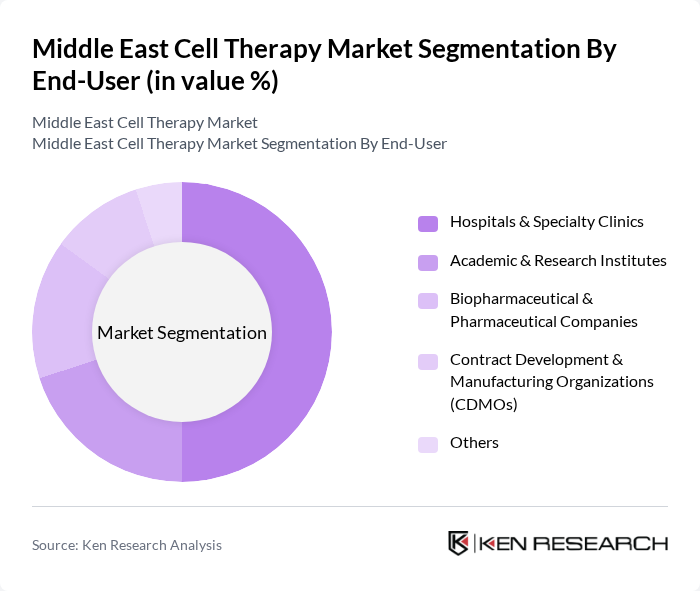
By End-User:The market is segmented by end-users, including Hospitals & Specialty Clinics, Academic & Research Institutes, Biopharmaceutical & Pharmaceutical Companies, Contract Development & Manufacturing Organizations (CDMOs), and Others. Hospitals & Specialty Clinics are the leading end-users due to their direct involvement in patient care and treatment administration, which drives demand for cell therapies. Specialty clinics and hospital pharmacies are also expanding their role in the distribution and administration of advanced cell therapies .
The Middle East Cell Therapy Market is characterized by a dynamic mix of regional and international players. Leading participants such as King Faisal Specialist Hospital & Research Centre (Saudi Arabia), Abu Dhabi Stem Cells Center (UAE), Lifera (Saudi Arabia), Pluristem Therapeutics Inc. (Israel), Gamida Cell Ltd. (Israel), Hadassah Medical Center (Israel), Orgenesis Inc. (Israel/UAE), Novartis AG, Gilead Sciences, Inc. (Kite Pharma), Bristol-Myers Squibb Company, Takeda Pharmaceutical Company Limited, CellGenix GmbH (A Sartorius Company), Miltenyi Biotec, Thermo Fisher Scientific Inc., Merck KGaA contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Middle East cell therapy market appears promising, driven by ongoing advancements in technology and increasing collaboration among stakeholders. As the region continues to invest in healthcare infrastructure, the accessibility of innovative therapies is expected to improve. Furthermore, the integration of artificial intelligence in research is likely to enhance the efficiency of cell therapy development, paving the way for more personalized treatment options that cater to individual patient needs and preferences.
| Segment | Sub-Segments |
|---|---|
| By Type | Autologous Cell Therapy Allogeneic Cell Therapy Stem Cell Therapy CAR-T Cell Therapy Mesenchymal Stem Cell Therapy Induced Pluripotent Stem Cell (iPSC) Therapy Others |
| By End-User | Hospitals & Specialty Clinics Academic & Research Institutes Biopharmaceutical & Pharmaceutical Companies Contract Development & Manufacturing Organizations (CDMOs) Others |
| By Application | Oncology (Hematologic Malignancies, Solid Tumors) Cardiovascular Diseases Neurological Disorders Autoimmune Diseases Rare Diseases Infectious Diseases Regenerative Medicine Others |
| By Source of Cells | Bone Marrow Peripheral Blood Umbilical Cord Blood Adipose Tissue Others |
| By Technology | Gene Editing Cell Expansion Technologies Cell Preservation Technologies Automated Cell Processing Platforms Others |
| By Distribution Channel | Direct Sales Online Sales Distributors Hospital Pharmacies Others |
| By Region | GCC Countries (Saudi Arabia, UAE, Qatar, Kuwait, Bahrain, Oman) Levant Region (Israel, Jordan, Lebanon, Iraq, Syria, Palestine) North Africa (Egypt, Morocco, Algeria, Tunisia) Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Oncology Cell Therapy Practices | 100 | Oncologists, Clinical Researchers |
| Hematology and Stem Cell Transplantation | 80 | Hematologists, Transplant Coordinators |
| Regenerative Medicine Clinics | 60 | Clinic Directors, Medical Practitioners |
| Patient Experience with Cell Therapies | 50 | Patients, Caregivers |
| Healthcare Policy Makers | 40 | Health Ministry Officials, Regulatory Affairs Specialists |
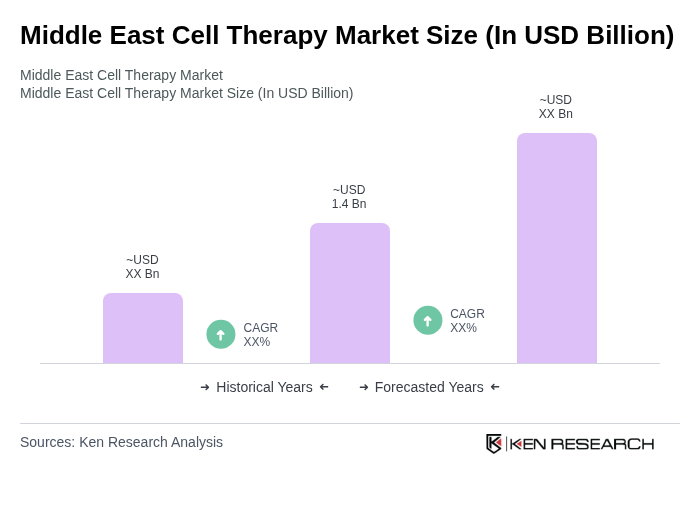
The Middle East Cell Therapy Market is valued at approximately USD 1.4 billion, driven by advancements in medical technology, increasing prevalence of chronic diseases, and rising investments in healthcare infrastructure.