Region:Asia
Author(s):Shubham
Product Code:KRAB3231
Pages:96
Published On:October 2025

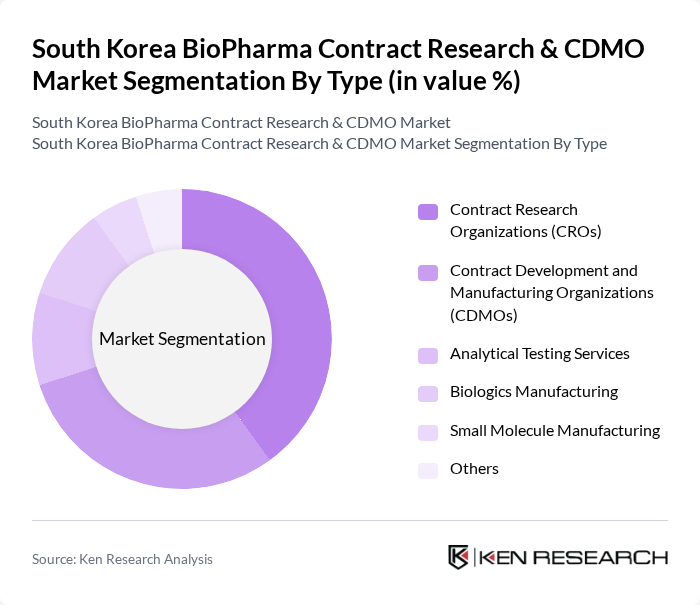
By Type:The market is segmented into various types, including Contract Research Organizations (CROs), Contract Development and Manufacturing Organizations (CDMOs), Analytical Testing Services, Biologics Manufacturing, Small Molecule Manufacturing, and Others. Among these, Contract Research Organizations (CROs) are leading the market due to their essential role in providing outsourced research services to pharmaceutical and biotechnology companies. The increasing complexity of clinical trials and the need for specialized expertise have driven demand for CROs, making them a critical component of the biopharma ecosystem.
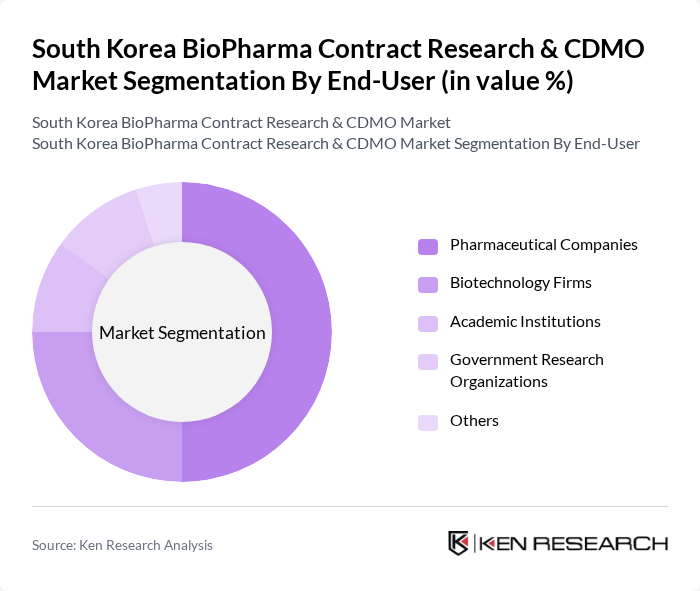
By End-User:The end-user segmentation includes Pharmaceutical Companies, Biotechnology Firms, Academic Institutions, Government Research Organizations, and Others. Pharmaceutical Companies are the dominant end-users, driven by their need for efficient drug development processes and the increasing complexity of regulatory requirements. The collaboration between pharmaceutical companies and CROs has become essential for accelerating drug development timelines and ensuring compliance with regulatory standards.
The South Korea BioPharma Contract Research & CDMO Market is characterized by a dynamic mix of regional and international players. Leading participants such as Samsung Biologics, SK Biopharmaceuticals, Celltrion, Hanmi Pharmaceutical, LG Chem, Daewoong Pharmaceutical, CJ Healthcare, Medytox, Genexine, Green Cross Corporation, PharmAbcine, ST Pharm, Yuhan Corporation, Dong-A ST, Ildong Pharmaceutical contribute to innovation, geographic expansion, and service delivery in this space.
The South Korean bioPharma contract research and CDMO market is poised for significant transformation, driven by advancements in personalized medicine and digital technologies. As the industry shifts towards patient-centric approaches, companies are increasingly adopting innovative methodologies to enhance clinical trial efficiency. Furthermore, collaborations with academic institutions are expected to foster research breakthroughs, enabling the development of novel therapies. This dynamic environment will likely attract more investments, positioning South Korea as a leading hub for biopharmaceutical innovation in the Asia-Pacific region.
| Segment | Sub-Segments |
|---|---|
| By Type | Contract Research Organizations (CROs) Contract Development and Manufacturing Organizations (CDMOs) Analytical Testing Services Biologics Manufacturing Small Molecule Manufacturing Others |
| By End-User | Pharmaceutical Companies Biotechnology Firms Academic Institutions Government Research Organizations Others |
| By Service Type | Preclinical Services Clinical Development Services Commercial Manufacturing Services Regulatory Affairs Services Others |
| By Therapeutic Area | Oncology Cardiovascular Neurology Infectious Diseases Others |
| By Region | Seoul Busan Incheon Daegu Others |
| By Client Type | Large Enterprises Small and Medium Enterprises (SMEs) Startups Others |
| By Pricing Model | Fixed Pricing Cost-Plus Pricing Performance-Based Pricing Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Contract Research Organizations | 100 | Business Development Managers, Project Managers |
| Contract Manufacturing Organizations | 80 | Operations Directors, Quality Assurance Managers |
| Biotech Startups | 60 | Founders, R&D Leads |
| Pharmaceutical Companies | 90 | Clinical Research Coordinators, Regulatory Affairs Managers |
| Academic Research Institutions | 70 | Principal Investigators, Lab Managers |
The South Korea BioPharma Contract Research & CDMO Market is valued at approximately USD 5 billion, driven by the increasing demand for biopharmaceuticals and advancements in drug development technologies.