Region:North America
Author(s):Geetanshi
Product Code:KRAD3917
Pages:94
Published On:November 2025
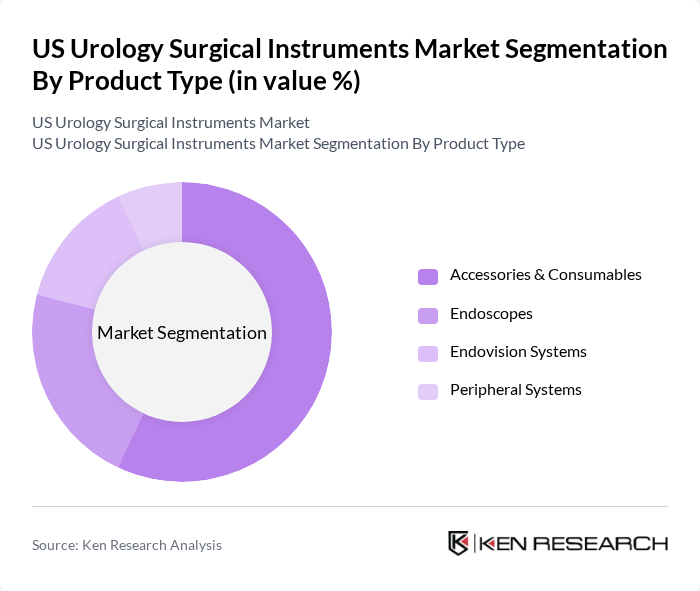
By Product Type:The product type segmentation includes various categories such as Endoscopes, Endovision Systems, Peripheral Systems, and Accessories & Consumables. Accessories & Consumables represent the leading sub-segment due to their recurring demand, critical role in minimally invasive surgeries, and the increasing preference for advanced imaging technologies. The growing trend towards outpatient procedures further bolsters the market for these essential consumable products.
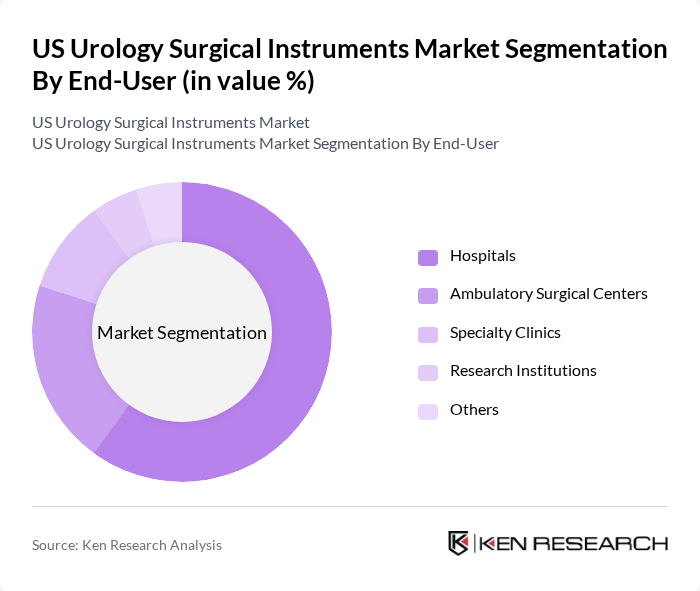
By End-User:The end-user segmentation encompasses Hospitals, Ambulatory Surgical Centers, Specialty Clinics, Research Institutions, and Others. Hospitals dominate this segment, accounting for a significant share due to their comprehensive facilities and the high volume of urological procedures performed. The increasing adoption of advanced surgical technologies in hospitals, along with the growing patient population, drives the demand for urology surgical instruments in this setting.
The US Urology Surgical Instruments Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic PLC, Boston Scientific Corporation, Olympus Corporation, Karl Storz GmbH & Co. KG, Richard Wolf GmbH, Stryker Corporation, Teleflex Incorporated, Coloplast A/S, Cook Medical, Johnson & Johnson (Ethicon), B. Braun Melsungen AG, ConMed Corporation, Intuitive Surgical, Inc., Merit Medical Systems, Inc., EndoChoice Holdings, Inc. contribute to innovation, geographic expansion, and service delivery in this space.
The future of the US urology surgical instruments market appears promising, driven by ongoing technological innovations and an increasing focus on patient-centered care. As healthcare providers continue to adopt robotic-assisted and minimally invasive surgical techniques, the demand for advanced instruments is expected to rise. Additionally, the integration of digital technologies, such as telemedicine and AI, will likely enhance surgical planning and execution, further shaping the market landscape in the future.
| Segment | Sub-Segments |
|---|---|
| By Product Type | Endoscopes Endovision Systems Peripheral Systems Accessories & Consumables |
| By End-User | Hospitals Ambulatory Surgical Centers Specialty Clinics Research Institutions Others |
| By Application | Urological Surgery Nephrology Oncology Pediatric Urology Others |
| By Material | Stainless Steel Plastic Titanium Others |
| By Distribution Channel | Direct Sales Distributors Online Sales Others |
| By Region | Northeast Midwest South West |
| By Product Lifecycle Stage | New Products Mature Products Declining Products Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Urology Surgical Instrument Procurement | 100 | Procurement Managers, Hospital Supply Chain Directors |
| Urologist Insights on Instrument Usage | 80 | Urologists, Surgical Specialists |
| Operating Room Equipment Managers | 60 | Operating Room Managers, Surgical Coordinators |
| Healthcare Facility Administrators | 50 | Healthcare Administrators, Financial Officers |
| Clinical Research on Urology Instruments | 40 | Clinical Researchers, Medical Device Analysts |
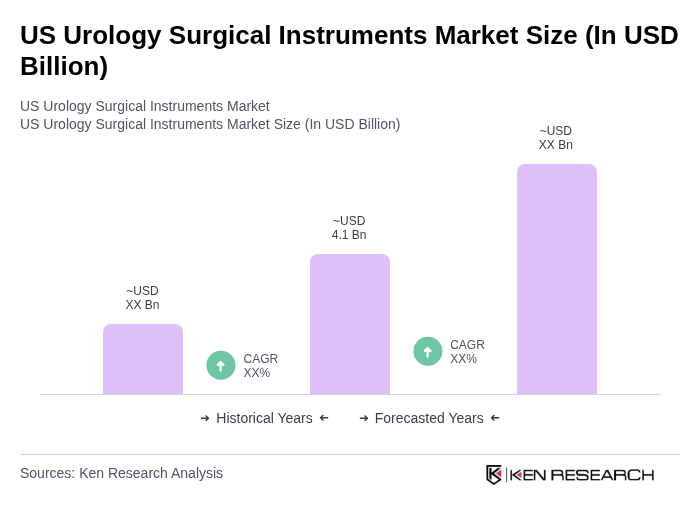
The US Urology Surgical Instruments Market is valued at approximately USD 4.1 billion, reflecting a significant growth driven by the rising prevalence of urological disorders, advancements in surgical technologies, and an increasing geriatric population requiring urological interventions.