Region:North America
Author(s):Geetanshi
Product Code:KRAD8214
Pages:90
Published On:December 2025
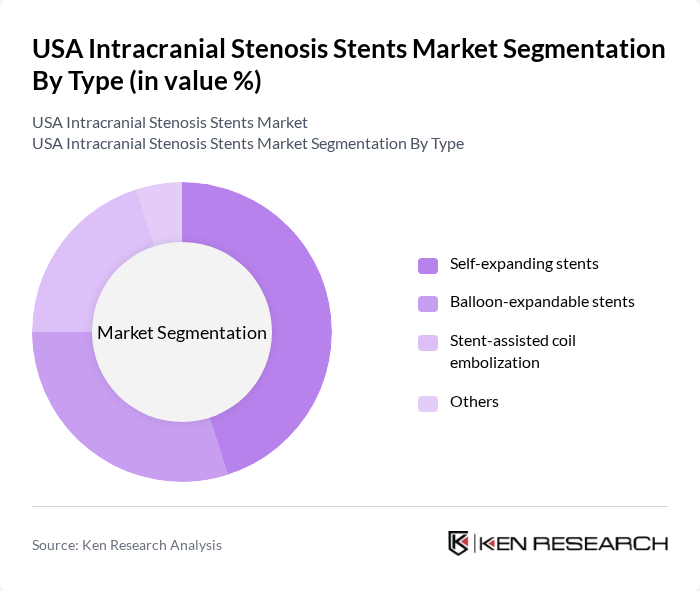
By Type:The market is segmented into various types of stents, including self-expanding stents, balloon-expandable stents, stent-assisted coil embolization, and others. Among these, self-expanding stents are gaining traction due to their ease of use and adaptability to different vessel diameters, making them a preferred choice for many clinicians. Balloon-expandable stents are also significant, particularly in acute settings where rapid deployment is crucial. The stent-assisted coil embolization segment is growing as it offers a combined approach for treating complex aneurysms.
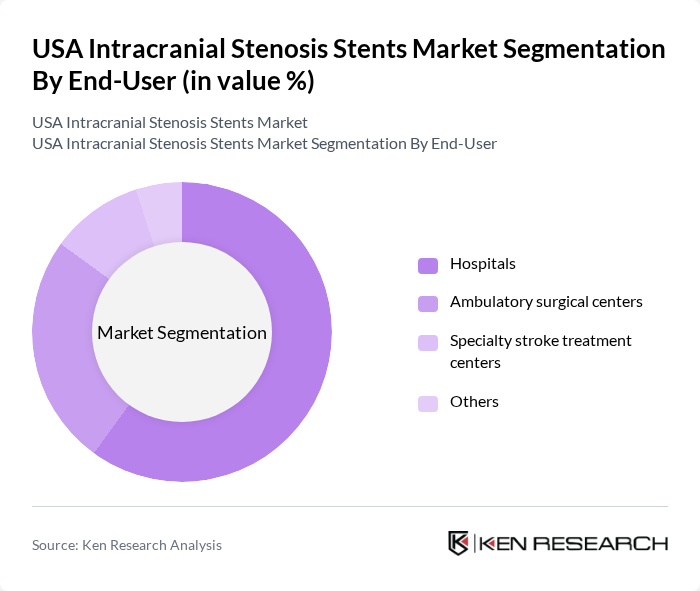
By End-User:The end-user segmentation includes hospitals, ambulatory surgical centers, specialty stroke treatment centers, and others. Hospitals are the dominant end-user segment due to their comprehensive facilities and access to advanced medical technologies. Ambulatory surgical centers are also witnessing growth as they provide cost-effective and efficient care for patients requiring stenting procedures. Specialty stroke treatment centers are increasingly recognized for their focused expertise in managing complex cases, further driving demand.
The USA Intracranial Stenosis Stents Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic, Boston Scientific, Stryker Corporation, Abbott Laboratories, Terumo Corporation, Johnson & Johnson, MicroVention (Terumo subsidiary), Penumbra, Inc., Cook Medical, B. Braun Melsungen AG, Cerenovus (Johnson & Johnson subsidiary), Asahi Intecc, Merit Medical Systems, Neuravi Limited, Endologix, Inc. contribute to innovation, geographic expansion, and service delivery in this space.
The future of the USA intracranial stenosis stents market appears promising, driven by ongoing technological advancements and an increasing focus on patient-centered care. As healthcare providers adopt minimally invasive techniques, the demand for innovative stent designs is expected to rise. Furthermore, the integration of digital health technologies will enhance patient monitoring and outcomes. Collaborations between manufacturers and healthcare providers will likely foster the development of tailored solutions, ensuring that the market adapts to evolving patient needs and preferences in the coming years.
| Segment | Sub-Segments |
|---|---|
| By Type | Self-expanding stents Balloon-expandable stents Stent-assisted coil embolization Others |
| By End-User | Hospitals Ambulatory surgical centers Specialty stroke treatment centers Others |
| By Disease Indication | Intracranial stenosis (primary indication) Brain aneurysm Intracranial atherosclerotic disease Others |
| By Patient Demographics | Age group (Adult, Geriatric) Gender Comorbidities (hypertension, diabetes, high cholesterol) Others |
| By Distribution Channel | Direct sales to hospitals Medical device distributors Specialty neurovascular distributors Others |
| By Geography | Northeast Midwest South West |
| By Clinical Application | Ischemic stroke prevention and treatment Transient ischemic attack (TIA) Acute stroke intervention Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Interventional Neurology Practices | 100 | Interventional Neurologists, Neurosurgeons |
| Hospital Administration | 80 | Healthcare Administrators, Procurement Managers |
| Patient Experience and Outcomes | 75 | Patients with Intracranial Stents, Caregivers |
| Medical Device Distributors | 60 | Sales Representatives, Distribution Managers |
| Clinical Research Institutions | 50 | Clinical Researchers, Medical Directors |
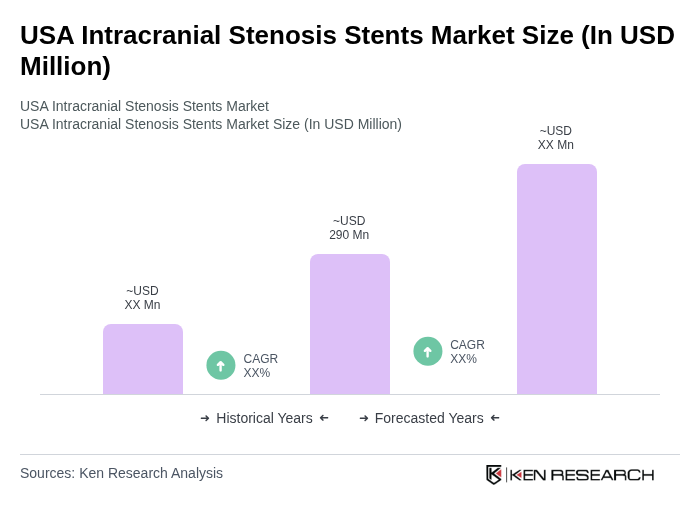
The USA Intracranial Stenosis Stents Market is valued at approximately USD 290 million, reflecting a significant growth driven by the rising prevalence of neurological disorders and advancements in stent technology.