Region:North America
Author(s):Geetanshi
Product Code:KRAD7944
Pages:87
Published On:December 2025

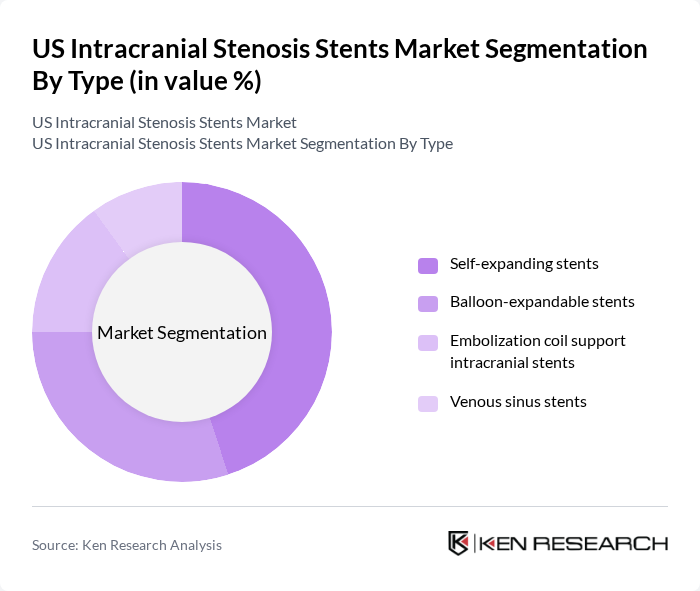
By Type:The market is segmented into various types of stents, including self-expanding stents, balloon-expandable stents, embolization coil support intracranial stents, and venous sinus stents. Among these, self-expanding stents are currently leading the market due to their ease of use and adaptability to the vascular anatomy. They are preferred for their ability to maintain patency in varying vessel diameters, which is crucial for effective treatment outcomes.
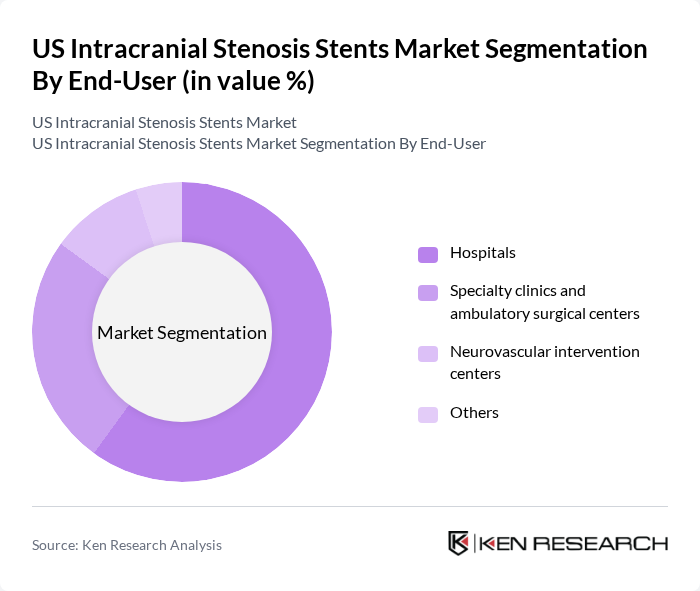
By End-User:The end-user segmentation includes hospitals, specialty clinics and ambulatory surgical centers, neurovascular intervention centers, and others. Hospitals dominate this segment due to their comprehensive facilities and access to advanced medical technologies. They are equipped to handle complex cases and provide a multidisciplinary approach to patient care, which is essential for effective treatment of intracranial stenosis.
The US Intracranial Stenosis Stents Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic, Stryker Corporation, Johnson & Johnson (CERENOVUS), MicroVention, Inc., Penumbra, Inc., Acandis GmbH, phenox GmbH, MicroPort Scientific Corporation, Sino Medical Sciences Technology, Inc., Balt, Cook Medical, Boston Scientific contribute to innovation, geographic expansion, and service delivery in this space.
The future of the US intracranial stenosis stents market appears promising, driven by ongoing technological advancements and an increasing focus on minimally invasive procedures. As healthcare providers continue to adopt innovative stenting techniques, patient outcomes are expected to improve significantly. Additionally, the integration of artificial intelligence in stent placement is likely to enhance procedural accuracy, further driving market growth. The emphasis on patient-centric care will also shape future developments in this sector, ensuring better access and outcomes for patients.
| Segment | Sub-Segments |
|---|---|
| By Type | Self-expanding stents Balloon-expandable stents Embolization coil support intracranial stents Venous sinus stents |
| By End-User | Hospitals Specialty clinics and ambulatory surgical centers Neurovascular intervention centers Others |
| By Patient Demographics | Age group (Adult, Geriatric) Gender Comorbidities (Hypertension, Diabetes, Atherosclerosis) Others |
| By Distribution Channel | Direct sales to hospitals and healthcare facilities Medical device distributors Online sales platforms Others |
| By Geography | Northeast Midwest South West |
| By Material | Nitinol (nickel-titanium) stents Stainless steel stents Polymer-coated stents Others |
| By Application | Intracranial atherosclerotic disease (ICAD) Intracranial atherosclerotic stroke (ICAS) Intracranial aneurysm treatment Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Neurosurgeons Specializing in Stenting | 45 | Neurosurgeons, Interventional Radiologists |
| Hospital Administrators | 40 | Healthcare Administrators, Procurement Managers |
| Patients with Intracranial Stenosis | 35 | Patients, Caregivers |
| Medical Device Distributors | 30 | Sales Representatives, Product Managers |
| Healthcare Policy Experts | 25 | Health Economists, Policy Analysts |
The US Intracranial Stenosis Stents Market is valued at approximately USD 281 million, reflecting a significant growth driven by the increasing prevalence of intracranial stenosis and advancements in stent technology.