Region:Middle East
Author(s):Shubham
Product Code:KRAD5479
Pages:99
Published On:December 2025
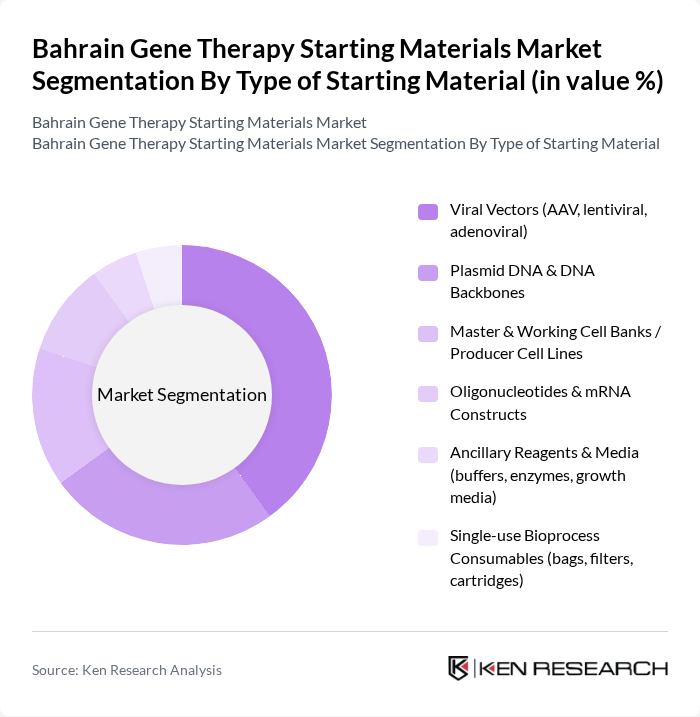
By Type of Starting Material:The market is segmented into various types of starting materials essential for gene therapy, including viral vectors, plasmid DNA, cell banks, oligonucleotides, ancillary reagents, and single-use bioprocess consumables. Among these, viral vectors are currently dominating the market due to their effectiveness in delivering therapeutic genes into target cells. The increasing number of clinical trials and the growing focus on gene therapies for rare diseases are driving the demand for these materials.
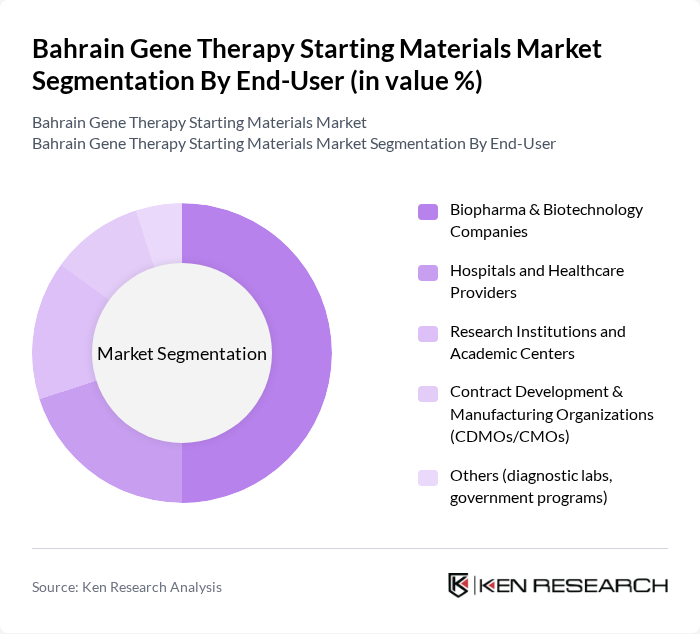
By End-User:The market is segmented by end-users, including biopharma and biotechnology companies, hospitals and healthcare providers, research institutions, and contract development organizations. Biopharma and biotechnology companies are the leading end-users, driven by their focus on developing innovative gene therapies and the increasing number of partnerships with research institutions for clinical trials.
The Bahrain Gene Therapy Starting Materials Market is characterized by a dynamic mix of regional and international players. Leading participants such as Novartis AG, Gilead Sciences, Inc., Spark Therapeutics, Inc., bluebird bio, Inc., Amgen Inc., Regeneron Pharmaceuticals, Inc., CRISPR Therapeutics AG, Editas Medicine, Inc., Intellia Therapeutics, Inc., Sarepta Therapeutics, Inc., F. Hoffmann?La Roche Ltd, Pfizer Inc., Johnson & Johnson, AbbVie Inc., Avernus Pharma (Bahrain) contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Bahrain gene therapy starting materials market appears promising, driven by ongoing advancements in biotechnology and increasing public awareness of genetic disorders. As research collaborations between academic institutions and industry players expand, innovative therapies are likely to emerge. Furthermore, the integration of artificial intelligence in research processes is expected to enhance the efficiency of gene therapy development, paving the way for more personalized treatment options that cater to individual patient needs.
| Segment | Sub-Segments |
|---|---|
| By Type of Starting Material | Viral Vectors (AAV, lentiviral, adenoviral) Plasmid DNA & DNA Backbones Master & Working Cell Banks / Producer Cell Lines Oligonucleotides & mRNA Constructs Ancillary Reagents & Media (buffers, enzymes, growth media) Single-use Bioprocess Consumables (bags, filters, cartridges) |
| By End-User | Biopharma & Biotechnology Companies Hospitals and Healthcare Providers Research Institutions and Academic Centers Contract Development & Manufacturing Organizations (CDMOs/CMOs) Others (diagnostic labs, government programs) |
| By Therapeutic Area | Rare Genetic Disorders Oncology (including CAR?T and oncolytic vectors) Neurological & Spinal Muscular Atrophy (SMA) Cardiovascular & Metabolic Diseases Autoimmune & Inflammatory Diseases Others |
| By Vector / Delivery Technology | Viral Vector Therapies Non?Viral Vectors CRISPR?based Gene Editing Systems Antisense Oligonucleotides & RNAi Others |
| By Manufacturing Scale & Service Model | Clinical?Scale Manufacturing Commercial?Scale Manufacturing Contract Manufacturing & Development Services Analytical & QC Services |
| By End-Use Setting in Bahrain | Central Bahrain (Manama) Northern Governorate Southern Governorate Muharraq Governorate |
| By Regulatory & Development Stage | Approved / Commercial Products Products Under Regulatory Review Clinical?Stage Programs Preclinical & Research?Grade Materials |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Gene Therapy Research Institutions | 45 | Research Scientists, Lab Managers |
| Biotechnology Firms in Bahrain | 40 | Product Development Managers, Regulatory Affairs Specialists |
| Healthcare Providers and Clinics | 35 | Clinical Researchers, Medical Directors |
| Government Health Agencies | 25 | Policy Makers, Health Program Coordinators |
| Investors in Biotechnology | 30 | Venture Capitalists, Financial Analysts |
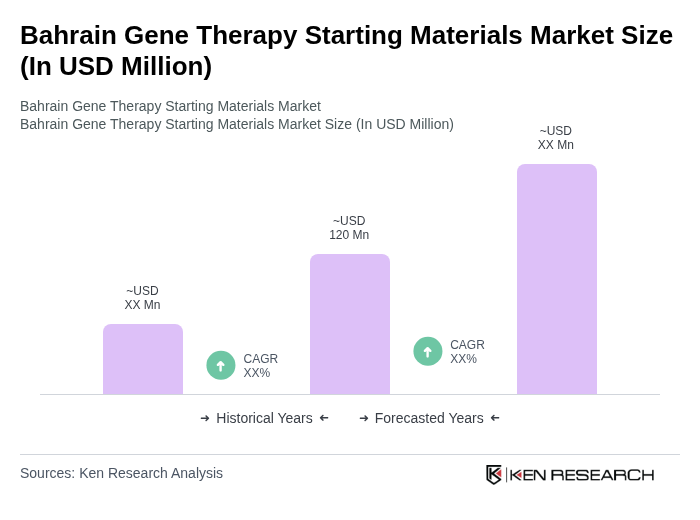
The Bahrain Gene Therapy Starting Materials Market is valued at approximately USD 120 million, reflecting significant growth driven by advancements in biotechnology, increased research investments, and a rising prevalence of genetic disorders.