Region:Asia
Author(s):Shubham
Product Code:KRAC0643
Pages:82
Published On:August 2025
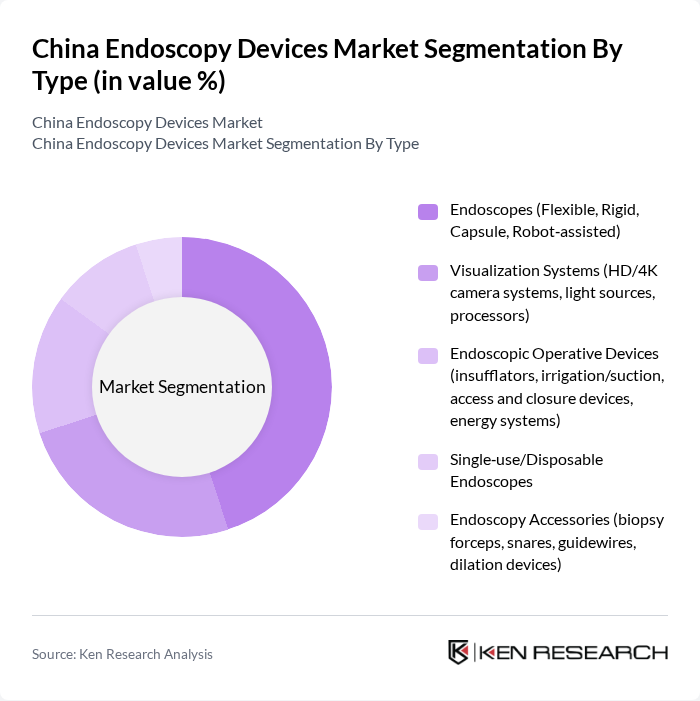
By Type:The market is segmented into various types of endoscopy devices, including endoscopes, visualization systems, endoscopic operative devices, single-use/disposable endoscopes, and endoscopy accessories. Among these, endoscopes, which include flexible, rigid, capsule, and robot-assisted types, are the most widely used due to their versatility and effectiveness in various medical applications. Visualization systems, which enhance the clarity and detail of images during procedures, are also gaining traction as they improve diagnostic accuracy.
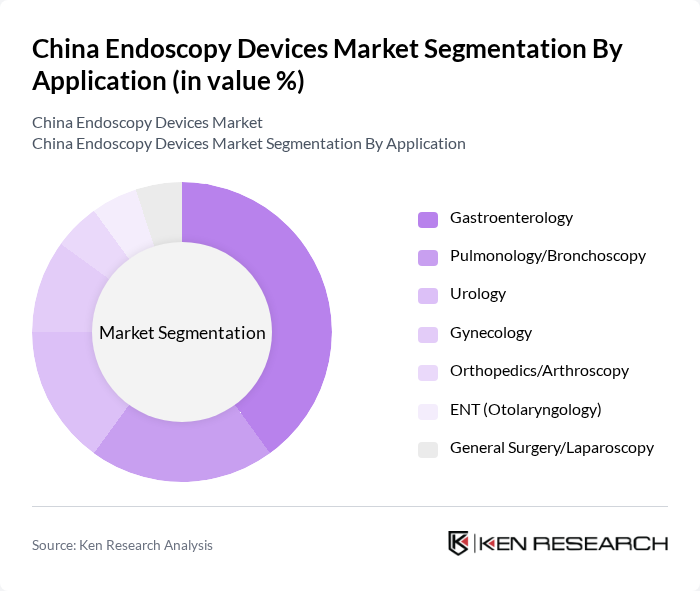
By Application:The applications of endoscopy devices span various medical fields, including gastroenterology, pulmonology, urology, gynecology, orthopedics, ENT, and general surgery. Gastroenterology is the leading application area, driven by the high prevalence of gastrointestinal disorders and the increasing adoption of endoscopic procedures for diagnosis and treatment. The demand for endoscopic solutions in pulmonology and urology is also on the rise, reflecting the growing focus on minimally invasive techniques in these specialties.
The China Endoscopy Devices Market is characterized by a dynamic mix of regional and international players. Leading participants such as Olympus Corporation, Medtronic plc, Boston Scientific Corporation, Stryker Corporation, KARL STORZ SE & Co. KG, FUJIFILM Holdings Corporation, HOYA Corporation (PENTAX Medical), CONMED Corporation, SonoScape Medical Corporation, Ambu A/S, Cook Medical LLC, B. Braun Melsungen AG, Richard Wolf GmbH, Mindray (Shenzhen Mindray Bio?Medical Electronics Co., Ltd.), EndoFresh Inc. (Shanghai AnKON Endoscopy/AnX Robotica China), Aohua Endoscopy (Shanghai Aohua Photoelectricity Endoscope Co., Ltd.), Jiangsu Vedkang Medical Science and Technology Co., Ltd. (Vedkang), Micro?Tech (Nanjing) Co., Ltd., United Imaging Healthcare (endoscopic visualization solutions), Chongqing Jinshan Science & Technology (AnKON capsule endoscopy) contribute to innovation, geographic expansion, and service delivery in this space.
The future of the China endoscopy devices market appears promising, driven by technological advancements and an increasing focus on patient-centric healthcare solutions. As the healthcare infrastructure continues to improve, particularly in rural areas, access to endoscopic procedures is expected to expand. Additionally, the integration of artificial intelligence in diagnostics and treatment planning will likely enhance procedural efficiency and accuracy, further propelling market growth in the coming years.
| Segment | Sub-Segments |
|---|---|
| By Type | Endoscopes (Flexible, Rigid, Capsule, Robot?assisted) Visualization Systems (HD/4K camera systems, light sources, processors) Endoscopic Operative Devices (insufflators, irrigation/suction, access and closure devices, energy systems) Single?use/Disposable Endoscopes Endoscopy Accessories (biopsy forceps, snares, guidewires, dilation devices) |
| By Application | Gastroenterology Pulmonology/Bronchoscopy Urology Gynecology Orthopedics/Arthroscopy ENT (Otolaryngology) General Surgery/Laparoscopy |
| By End-User | Tertiary Hospitals (Class III) Secondary Hospitals (Class II) Ambulatory Surgery Centers Specialty Clinics |
| By Distribution Channel | Direct Sales to Hospitals National/Regional Distributors Online Procurement Platforms (e.g., provincial volume?based procurement portals) Group Purchasing Organizations (GPOs) |
| By Region | East China (e.g., Shanghai, Jiangsu, Zhejiang, Shandong) North China (e.g., Beijing, Tianjin, Hebei, Shanxi) South China (e.g., Guangdong, Fujian, Hainan) West China (e.g., Sichuan, Chongqing, Shaanxi, Yunnan, Xinjiang) |
| By Price Range | Value Segment Mid?Range Segment Premium Segment |
| By Technology | Digital/Video Endoscopy (HD/4K, NBI/i?SCAN/BLI) Optical Endoscopy Robotic/Robot?assisted Endoscopy AI?assisted Computer?Aided Detection/Diagnosis (CADe/CADx) |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Gastroenterology Clinics | 90 | Gastroenterologists, Clinic Managers |
| General Hospitals | 120 | Surgeons, Procurement Officers |
| Medical Device Distributors | 80 | Sales Managers, Product Specialists |
| Healthcare Policy Makers | 50 | Health Economists, Regulatory Affairs Managers |
| Endoscopy Equipment Manufacturers | 70 | Product Development Managers, Marketing Directors |
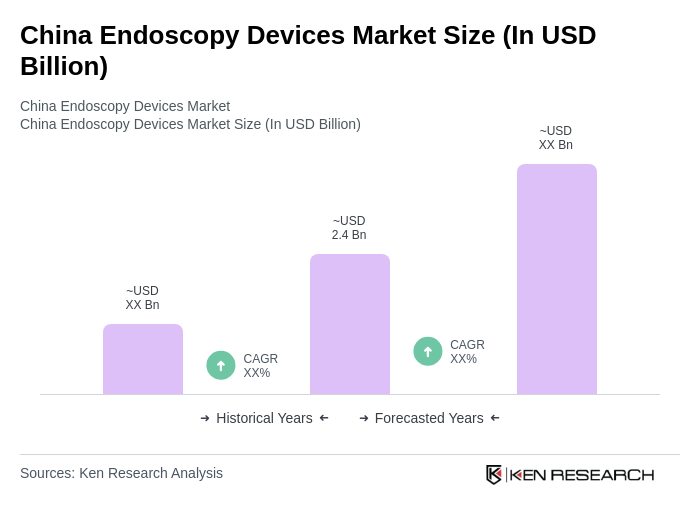
The China Endoscopy Devices Market is valued at approximately USD 2.4 billion, reflecting a significant growth driven by the increasing prevalence of chronic diseases and advancements in endoscopic technologies.