Region:Global
Author(s):Geetanshi
Product Code:KRAA2824
Pages:80
Published On:August 2025
By Type:The market is segmented into various types of amniotic membranes, including Cryopreserved Amniotic Membrane, Dehydrated Amniotic Membrane, Amniotic Membrane Grafts, Lyophilized Amniotic Membrane, and Others. Among these, Cryopreserved Amniotic Membrane is currently the leading subsegment due to its superior preservation of biological properties, high effectiveness in ocular and wound care procedures, and increasing demand for high-quality grafts in surgical applications .

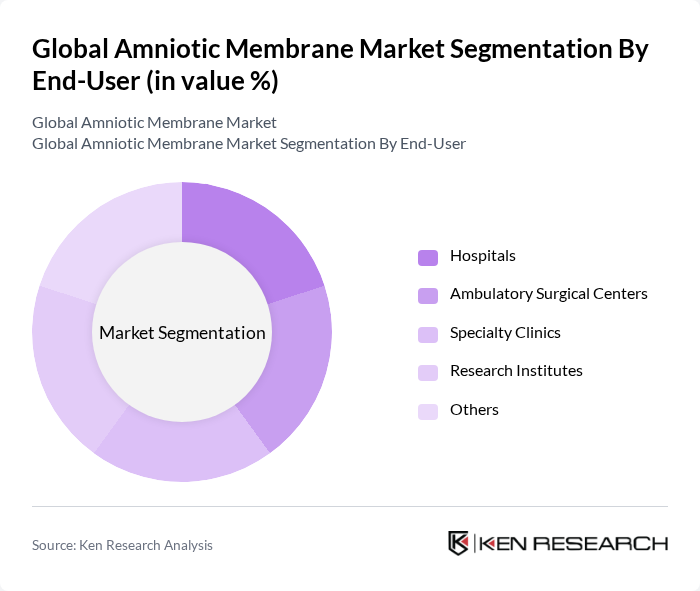
By End-User:The end-user segmentation includes Hospitals, Ambulatory Surgical Centers, Specialty Clinics, Research Institutes, and Others. Hospitals are the dominant end-user segment, primarily due to their extensive surgical capabilities, the high volume of wound care and ophthalmic procedures, and the adoption of advanced tissue-based therapies for regenerative medicine .
The Global Amniotic Membrane Market is characterized by a dynamic mix of regional and international players. Leading participants such as Amniox Medical, Inc., MiMedx Group, Inc., AlloSource, Organogenesis Inc., Tissue Regenix Group plc, Integra LifeSciences Corporation, Stryker Corporation, Human Regenerative Technologies, LLC, Osiris Therapeutics, Inc., Acelity L.P. Inc., Advanced BioHealing, Inc., Vericel Corporation, Medline Industries, Inc., MTF Biologics, TissueTech, Inc., Alliqua BioMedical, Inc., Amnio Technology, LLC, Applied Biologics LLC, DermaSciences, Katena Products, Inc., Skye Biologics, Inc., and Eliksa Therapeutics contribute to innovation, geographic expansion, and service delivery in this space .
The future of the amniotic membrane market appears promising, driven by ongoing advancements in regenerative medicine and increasing applications across various medical fields. As healthcare systems continue to prioritize innovative wound care solutions, the integration of amniotic membranes into standard treatment protocols is likely to expand. Additionally, the rise of telemedicine is expected to facilitate remote consultations, enhancing patient access to specialized wound care services and driving market growth in the coming years.
| Segment | Sub-Segments |
|---|---|
| By Type | Cryopreserved Amniotic Membrane Dehydrated Amniotic Membrane Amniotic Membrane Grafts Lyophilized Amniotic Membrane Others |
| By End-User | Hospitals Ambulatory Surgical Centers Specialty Clinics Research Institutes Others |
| By Application | Wound Care (Chronic & Acute Wounds) Ophthalmology (Corneal & Ocular Surface Disorders) Orthopedics (Sports Injuries, Tendon Repair) Plastic Surgery Others |
| By Distribution Channel | Direct Sales Distributors Online Sales Hospital Procurement Others |
| By Region | North America (U.S., Canada, Mexico) Europe (Germany, U.K., France, Italy, Spain, Rest of Europe) Asia-Pacific (China, Japan, India, Australia, South Korea, Rest of Asia-Pacific) Latin America (Brazil, Argentina, Rest of Latin America) Middle East & Africa (South Africa, Saudi Arabia, UAE, Rest of MEA) |
| By Product Form | Sheets Suspensions Gels Powders Others |
| By Pricing Strategy | Premium Pricing Competitive Pricing Value-Based Pricing Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Ophthalmology Applications | 100 | Ophthalmologists, Clinic Managers |
| Dermatology Applications | 80 | Dermatologists, Medical Directors |
| Orthopedic Applications | 60 | Orthopedic Surgeons, Rehabilitation Specialists |
| Research Institutions | 40 | Research Scientists, Clinical Trial Coordinators |
| Healthcare Procurement | 90 | Procurement Officers, Supply Chain Managers |
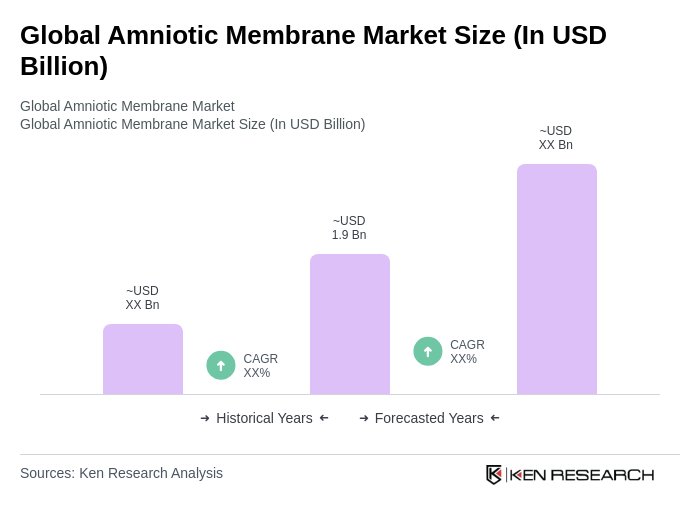
The Global Amniotic Membrane Market is valued at approximately USD 1.9 billion, driven by factors such as the increasing prevalence of chronic wounds, advancements in surgical techniques, and a growing demand for regenerative medicine.