Global Bionic Eyes Market Overview
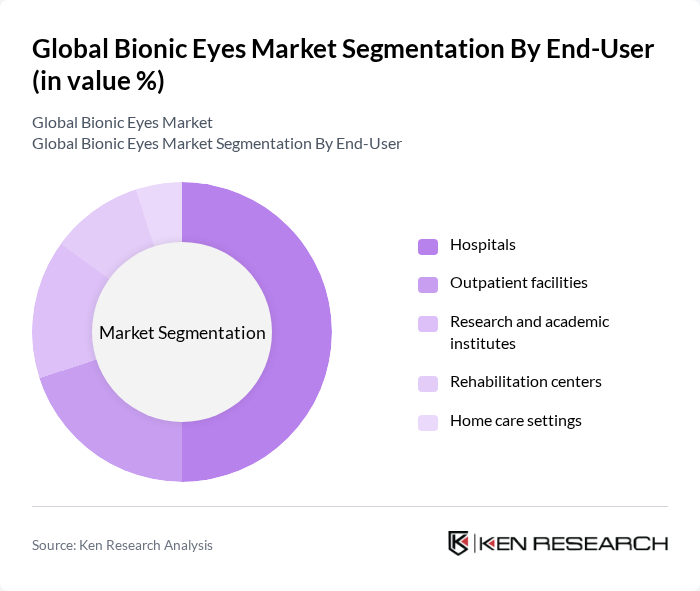
- The Global Bionic Eyes Market is valued at USD 320 million, based on a five-year historical analysis. This growth is primarily driven by advancements in neuroprosthetics, increasing prevalence of vision-related disorders such as retinitis pigmentosa and age-related macular degeneration, and rising investments in bioelectronics and research and development. The demand for innovative solutions to restore vision continues to expand, with a focus on improving the quality of life for visually impaired individuals through cutting-edge technologies and enhanced accessibility.
- Key players in this market include the United States, Germany, and Japan, which dominate due to their strong healthcare infrastructure, high levels of investment in medical technology, and robust research and development activities. These countries are at the forefront of innovation in bionic eye technology, contributing significantly to the overall growth and development of the market. The United Kingdom and Australia are also notable contributors, driven by expanding healthcare infrastructure and active research partnerships.
- In 2023, the U.S. Food and Drug Administration (FDA) issued the “Guidance for Industry and Food and Drug Administration Staff: Clinical Performance Assessment of Implantable Visual Prostheses,” establishing new guidelines for the clinical evaluation of bionic eye devices. This regulation, published by the Center for Devices and Radiological Health, aims to streamline the approval process for innovative vision restoration technologies, ensuring that they meet safety and efficacy standards while promoting faster access to market for patients in need.
Global Bionic Eyes Market Segmentation
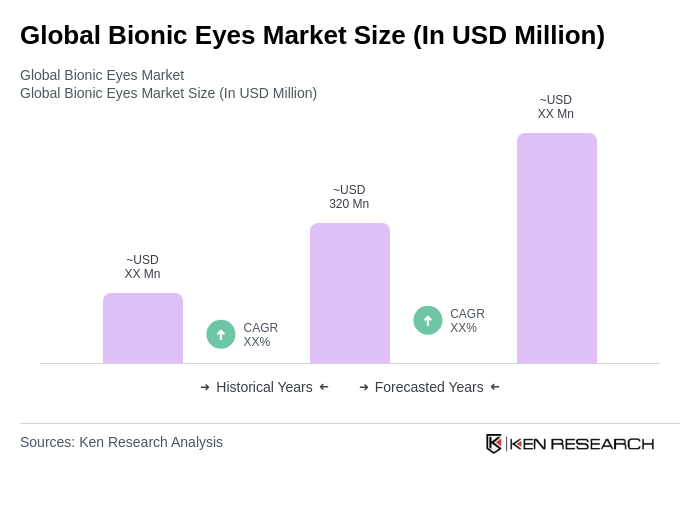
By Type:The bionic eyes market is segmented into various types, including retinal implants, cortical implants, optic nerve implants, external bionic eye systems, and others. Among these,retinal implantsare currently the most dominant segment due to their effectiveness in treating retinal diseases and their ability to restore partial vision. The increasing number of clinical trials and successful outcomes associated with retinal implants have significantly boosted their adoption in clinical settings. Cortical and optic nerve implants are gaining traction as research advances, while external bionic eye systems remain relevant for non-surgical applications.
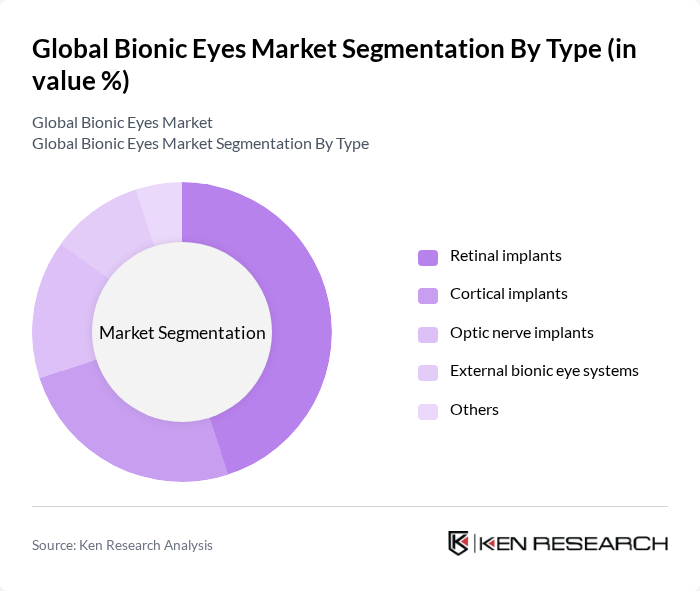
By End-User:The market is further segmented by end-user, including hospitals, outpatient facilities, research and academic institutes, rehabilitation centers, and home care settings.Hospitalsare the leading end-user segment, primarily due to their advanced medical facilities and access to specialized healthcare professionals. The increasing number of surgeries and treatments performed in hospitals has driven the demand for bionic eye technologies, making them a critical component of patient care. Outpatient facilities and research institutes are also important, reflecting the expanding scope of clinical research and post-operative rehabilitation.
Global Bionic Eyes Market Competitive Landscape
The Global Bionic Eyes Market is characterized by a dynamic mix of regional and international players. Leading participants such as Second Sight Medical Products, Inc., Bionic Vision Technologies Pty Ltd, Retina Implant AG, Pixium Vision S.A., Nano Retina Ltd., iBionics, Bionic Eye Technologies, Inc., Medtronic plc, Abbott Laboratories, Alcon Inc., Santen Pharmaceutical Co., Ltd., Carl Zeiss AG, NIDEK Co., Ltd., Optobionics Corporation, Visus Technology Inc. contribute to innovation, geographic expansion, and service delivery in this space.
Global Bionic Eyes Market Industry Analysis
Growth Drivers
- Increasing Prevalence of Visual Impairments:The World Health Organization estimates that approximately2.2 billionpeople globally suffer from some form of visual impairment. In None, this figure is projected to reach15 million, driven by an aging population and rising incidences of diabetes-related eye diseases. This growing patient base is expected to significantly boost the demand for bionic eye solutions, as healthcare providers seek innovative ways to address these challenges effectively.
- Advancements in Bionic Technology:Technological innovations in bionic eyes, such as improved image processing and neural interfacing, are enhancing the functionality and effectiveness of these devices. In None, investments in R&D are projected to exceed$500 million, facilitating breakthroughs that improve visual acuity and user experience. These advancements are crucial in attracting both patients and healthcare providers, thereby driving market growth and adoption rates for bionic eye implants.
- Rising Healthcare Expenditure:Healthcare spending in None is expected to reach$200 billion, reflecting a 5% increase from the previous year. This rise is attributed to government initiatives aimed at improving healthcare access and quality. As healthcare budgets expand, more resources will be allocated to advanced medical technologies, including bionic eyes, thereby fostering an environment conducive to market growth and innovation in this sector.
Market Challenges
- High Cost of Bionic Eye Implants:The average cost of bionic eye implants in None is around$100,000, which poses a significant barrier to accessibility for many potential users. This high price point limits the market to affluent patients or those with comprehensive insurance coverage. As a result, the overall adoption rate remains low, hindering the potential growth of the bionic eyes market in the region despite increasing demand.
- Limited Awareness Among Potential Users:A significant portion of the population in None remains unaware of the availability and benefits of bionic eye technology. Surveys indicate that only30%of individuals with visual impairments have heard of bionic solutions. This lack of awareness can lead to underutilization of available technologies, stalling market growth and preventing advancements from reaching those who could benefit most from these innovations.
Global Bionic Eyes Market Future Outlook
The future of the bionic eyes market in None appears promising, driven by ongoing technological advancements and increasing healthcare investments. As awareness grows and more patients seek innovative solutions for visual impairments, the market is likely to expand. Additionally, collaborations between technology firms and healthcare providers will foster the development of next-generation bionic eyes, enhancing their functionality and accessibility. This synergy is expected to create a robust ecosystem that supports sustained growth in the coming years.
Market Opportunities
- Expansion into Emerging Markets:There is a significant opportunity for bionic eye manufacturers to expand into emerging markets within None, where the prevalence of visual impairments is rising. By tailoring products to meet local needs and establishing partnerships with regional healthcare providers, companies can tap into a growing customer base, potentially increasing market penetration and revenue.
- Collaborations with Research Institutions:Collaborating with research institutions can accelerate the development of innovative bionic eye technologies. By leveraging academic expertise and resources, companies can enhance their R&D capabilities, leading to breakthroughs that improve device performance and patient outcomes. Such partnerships are essential for driving innovation and maintaining a competitive edge in the evolving bionic eyes market.