Global Cystic Fibrosis Therapeutics Market Overview
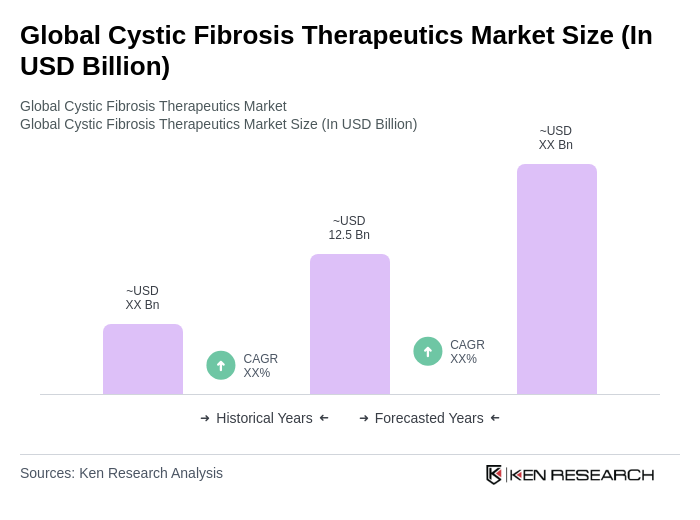
- The Global Cystic Fibrosis Therapeutics Market is valued at USD 12.5 billion, based on a five-year historical analysis. This growth is primarily driven by advancements in drug development, increased awareness of cystic fibrosis, and the rising prevalence of the disease globally. The introduction of innovative therapies, particularly CFTR modulators, has significantly improved patient outcomes, leading to higher demand for effective treatment options. Recent trends also highlight the emergence of gene therapies, RNA-based treatments, and digital health tools that enhance patient monitoring and personalized care, further supporting market expansion .
- Key players in this market are predominantly located in North America and Europe, with the United States and Germany being the most dominant countries. The presence of advanced healthcare infrastructure, significant investment in research and development, and a high prevalence of cystic fibrosis in these regions contribute to their market leadership. Additionally, supportive government policies, robust reimbursement frameworks, and a strong focus on patient care further enhance their position in the market .
- In 2023, the U.S. Food and Drug Administration (FDA) implemented the “Expedited Programs for Serious Conditions – Drugs and Biologics” guidance, which includes accelerated approval, breakthrough therapy designation, and priority review for cystic fibrosis therapies. This regulatory framework, issued by the U.S. Food and Drug Administration, facilitates faster approval of drugs that demonstrate substantial clinical benefit, thereby enhancing patient access to innovative treatments and encouraging pharmaceutical companies to invest in research and development .
Global Cystic Fibrosis Therapeutics Market Segmentation
By Type:The market is segmented into various types of therapeutics, including CFTR modulators, antibiotics, mucolytics, anti-inflammatory agents, nutritional supplements, bronchodilators, pancreatic enzyme supplements, and others. Among these, CFTR modulators are leading the market due to their targeted action on the underlying cause of cystic fibrosis, significantly improving lung function and quality of life for patients. The increasing adoption of these therapies, driven by their efficacy and the growing patient population, is a key factor in their dominance. Recent years have also seen the introduction of new drug classes such as ENaC inhibitors and gene therapies, expanding treatment options for patients unresponsive to traditional therapies .
By End-User:The end-user segmentation includes hospitals, specialty clinics, home care settings, and research institutions. Hospitals are the leading end-user segment, primarily due to their capacity to provide comprehensive care and access to advanced treatment options for cystic fibrosis patients. The increasing number of hospital admissions for cystic fibrosis-related complications and the availability of specialized care in these facilities contribute to their dominance in the market. Specialty clinics and home care settings are also gaining traction as multidisciplinary care models and remote monitoring technologies become more prevalent .
Global Cystic Fibrosis Therapeutics Market Competitive Landscape
The Global Cystic Fibrosis Therapeutics Market is characterized by a dynamic mix of regional and international players. Leading participants such as Vertex Pharmaceuticals Incorporated, AbbVie Inc., Gilead Sciences, Inc., Novartis AG, Roche Holding AG, Teva Pharmaceutical Industries Ltd., Chiesi Farmaceutici S.p.A., Sanofi S.A., AstraZeneca PLC, Merck & Co., Inc., Pfizer Inc., Eli Lilly and Company, Amgen Inc., Biogen Inc., Galapagos NV, Viatris Inc., Zambon S.p.A., Insmed Incorporated, Sionna Therapeutics, Translate Bio (now part of Sanofi) contribute to innovation, geographic expansion, and service delivery in this space.
Global Cystic Fibrosis Therapeutics Market Industry Analysis
Growth Drivers
- Increasing Prevalence of Cystic Fibrosis:The prevalence of cystic fibrosis (CF) is rising, with approximately 30,000 individuals affected in the United States alone. Globally, the incidence is about 1 in 3,500 live births, particularly in Caucasian populations. This growing patient population drives demand for effective therapies. According to the Cystic Fibrosis Foundation, the number of patients receiving care has increased by 25% over the last decade, highlighting the urgent need for innovative treatment solutions.
- Advancements in Gene Therapy:Significant advancements in gene therapy are transforming CF treatment paradigms. In future, Vertex Pharmaceuticals reported a breakthrough in gene-editing techniques, showing a 50% improvement in lung function in clinical trials. The global gene therapy market is projected to reach $13.3 billion by 2024, driven by innovations targeting genetic disorders like CF. These advancements not only enhance treatment efficacy but also attract substantial investments, further propelling market growth.
- Increased Funding for Research and Development:Funding for CF research has surged, with the National Institutes of Health allocating over $50 million in future for CF-related studies. Additionally, private sector investments have reached approximately $1 billion, fostering innovation in drug development. This financial support is crucial for advancing new therapies and improving existing treatment options, ultimately enhancing patient outcomes and driving market expansion in the CF therapeutics sector.
Market Challenges
- High Cost of Therapies:The cost of cystic fibrosis therapies remains a significant barrier, with annual treatment expenses exceeding $300,000 per patient for advanced therapies like Trikafta. This high cost limits accessibility for many patients, particularly in low-income regions. According to the World Health Organization, approximately 80% of patients in developing countries lack access to essential CF treatments, highlighting the urgent need for cost-effective solutions in the market.
- Regulatory Hurdles:Navigating regulatory frameworks poses challenges for CF therapeutics. The average time for drug approval can exceed 10 years, with stringent requirements for clinical trials. In future, the FDA rejected several applications for new CF drugs due to insufficient data on long-term safety. These regulatory hurdles can delay market entry for innovative therapies, impacting patient access and overall market growth in the cystic fibrosis sector.
Global Cystic Fibrosis Therapeutics Market Future Outlook
The future of cystic fibrosis therapeutics is poised for significant advancements, driven by ongoing research and technological innovations. The integration of digital health solutions is expected to enhance patient monitoring and adherence to therapies, improving overall outcomes. Additionally, the trend towards personalized medicine will likely lead to tailored treatment plans, optimizing efficacy for individual patients. As the market evolves, collaboration between pharmaceutical companies and biotech firms will be crucial in addressing unmet needs and expanding access to therapies.
Market Opportunities
- Expansion into Emerging Markets:Emerging markets present substantial opportunities for growth, with increasing healthcare investments. Countries like India and Brazil are witnessing rising CF diagnosis rates, creating demand for affordable therapies. The global healthcare expenditure in these regions is projected to reach $1.5 trillion by 2024, providing a fertile ground for market expansion and increased access to cystic fibrosis treatments.
- Development of Combination Therapies:The development of combination therapies is gaining traction, with studies indicating that multi-drug regimens can enhance treatment efficacy. In future, clinical trials demonstrated a 40% improvement in lung function with combination therapies. This approach not only addresses various disease mechanisms but also opens new revenue streams for pharmaceutical companies, positioning them favorably in the competitive landscape of cystic fibrosis therapeutics.