Region:Global
Author(s):Geetanshi
Product Code:KRAD5971
Pages:83
Published On:December 2025
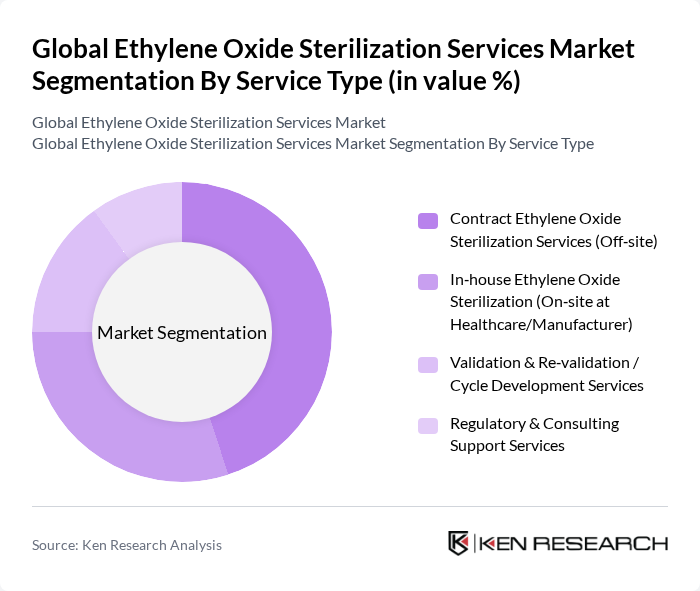
By Service Type:The service type segmentation includes various applications of ethylene oxide sterilization, each catering to different operational needs and client preferences. The dominant sub-segment is Contract Ethylene Oxide Sterilization Services (Off-site), which is preferred by many healthcare providers and medical device manufacturers due to its cost-effectiveness, scalability, and the ability to handle large volumes of sterilization without the need for in-house facilities. In-house Ethylene Oxide Sterilization (On-site) is also gaining traction as large healthcare facilities and manufacturers seek to maintain control over turnaround times, supply chain resilience, and proprietary processes. Validation & Re-validation / Cycle Development Services and Regulatory & Consulting Support Services are essential for ensuring compliance with standards such as ISO 11135, optimizing sterilization cycles, and meeting evolving environmental and safety requirements across different regions.
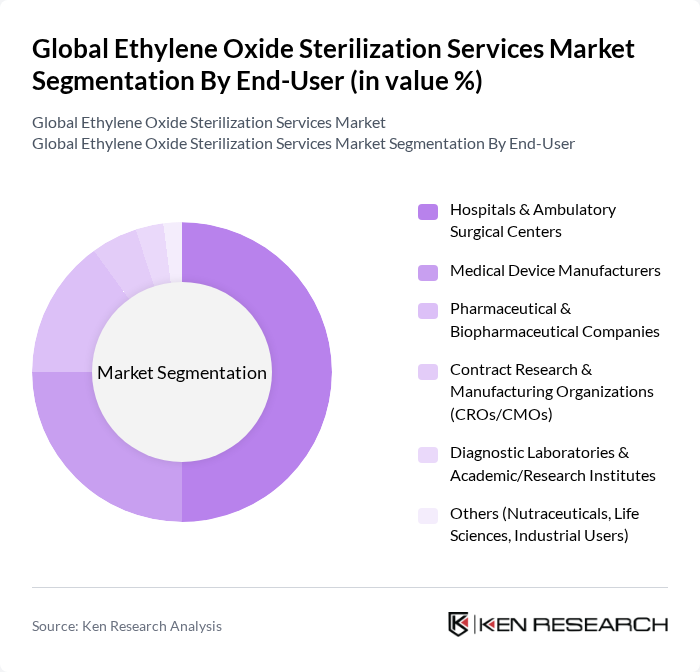
By End-User:The end-user segmentation highlights the various sectors utilizing ethylene oxide sterilization services. Hospitals & Ambulatory Surgical Centers dominate this segment due to their high demand for sterilized medical devices, single?use procedure kits, and instruments used in surgery and interventional procedures. Medical Device Manufacturers also represent a significant portion, as they rely heavily on ethylene oxide for heat- and moisture-sensitive products and outsource a substantial share of volumes to specialized contract sterilization providers to ensure product safety and compliance with regulatory standards. Pharmaceutical & Biopharmaceutical Companies, along with Contract Research & Manufacturing Organizations (CROs/CMOs), are increasingly relying on these services for components, packaging, and combination products where ethylene oxide is compatible, to maintain the integrity and sterility assurance of their products. Diagnostic Laboratories & Academic/Research Institutes, along with other sectors like Nutraceuticals, life sciences consumables, and selected industrial users, also contribute to the market as they adopt higher infection prevention standards and more complex disposable devices.
The Global Ethylene Oxide Sterilization Services Market is characterized by a dynamic mix of regional and international players. Leading participants such as STERIS plc (STERIS AST), Sotera Health Company and its subsidiary Sterigenics U.S., LLC, 3M Company, Getinge AB, Andersen Sterilizers, Inc., Cosmed Group, Inc., Ionisos SAS, Medistri SA, Noxilizer, Inc., E-BEAM Services, Inc., Becton, Dickinson and Company (BD), Cantel Medical LLC (A STERIS Company), Amsino International, Inc., TSO3 Inc., and Bioquell PLC (Ecolab Inc.) contribute to innovation, geographic expansion, and service delivery in this space through investments in new EtO capacity, emission-control upgrades, and integrated sterilization and monitoring solutions.
The future of ethylene oxide sterilization services appears promising, driven by technological advancements and a growing emphasis on infection control. As healthcare facilities increasingly adopt automated sterilization processes, efficiency and safety will improve. Additionally, the integration of IoT technologies for real-time monitoring will enhance operational effectiveness. These trends, coupled with rising investments in healthcare infrastructure, are expected to create a robust environment for the growth of ethylene oxide sterilization services in the coming years.
| Segment | Sub-Segments |
|---|---|
| By Service Type | Contract Ethylene Oxide Sterilization Services (Off?site) In?house Ethylene Oxide Sterilization (On?site at Healthcare/Manufacturer) Validation & Re?validation / Cycle Development Services Regulatory & Consulting Support Services |
| By End-User | Hospitals & Ambulatory Surgical Centers Medical Device Manufacturers Pharmaceutical & Biopharmaceutical Companies Contract Research & Manufacturing Organizations (CROs/CMOs) Diagnostic Laboratories & Academic/Research Institutes Others (Nutraceuticals, Life Sciences, Industrial Users) |
| By Application | Single?use Medical Devices (catheters, syringes, tubing, etc.) Complex & Implantable Devices Pharmaceutical & Biologic Products and Packaging Surgical Instruments & Reusable Equipment Laboratory Instruments, Consumables & Components Other Heat- & Moisture?sensitive Products |
| By Geography | North America Europe Asia-Pacific Latin America Middle East & Africa |
| By Mode of Delivery | Off?site (Dedicated Contract Sterilization Facilities) On?site (In?house Installed EtO Systems) Hybrid / Integrated Supply Chain Models |
| By Sterilization Cycle Type | Standard Ethylene Oxide Gas Cycles Low?temperature / Low?concentration EtO Cycles Accelerated / High?throughput EtO Cycles Custom Validated Cycles for Complex Devices |
| By Compliance & Certification Level | ISO 11135?compliant EtO Sterilization Services FDA?registered / CE?marked Supply Chain Providers cGMP?compliant Facilities Others (local regulatory & accreditation schemes) |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Hospital Sterilization Services | 120 | Sterilization Managers, Infection Control Officers |
| Medical Device Manufacturers | 90 | Quality Assurance Managers, Production Supervisors |
| Pharmaceutical Companies | 70 | Regulatory Affairs Specialists, Supply Chain Managers |
| Research Laboratories | 60 | Laboratory Managers, Safety Officers |
| Contract Sterilization Providers | 80 | Operations Directors, Business Development Managers |
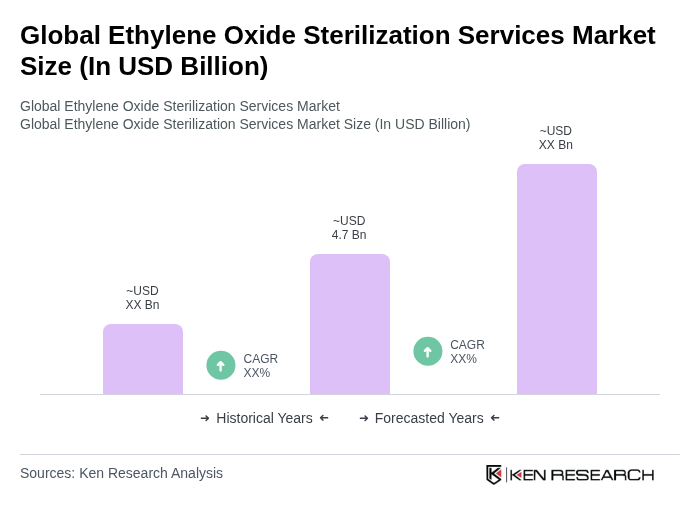
The Global Ethylene Oxide Sterilization Services Market is valued at approximately USD 4.7 billion, reflecting a significant demand for sterilization services, particularly in the healthcare sector for medical devices and pharmaceuticals.