Region:Global
Author(s):Geetanshi
Product Code:KRAC2303
Pages:85
Published On:October 2025
By Service Type:The service type segmentation includes various testing services essential for ensuring the safety and efficacy of medical devices. The subsegments are Preclinical Testing Services, Clinical Testing Services, Post-Market Surveillance Testing, Regulatory Compliance Testing, Biocompatibility Testing, Sterilization Validation, Package Testing, Chemical Testing, Electrical Safety Testing, and Mechanical Testing. Among these, Clinical Testing Services dominate the market due to the increasing number of clinical trials and the need for rigorous testing protocols to meet regulatory requirements. Recent trends also highlight rapid growth in biocompatibility and chemistry testing, driven by the complexity of device materials and regulatory expectations .

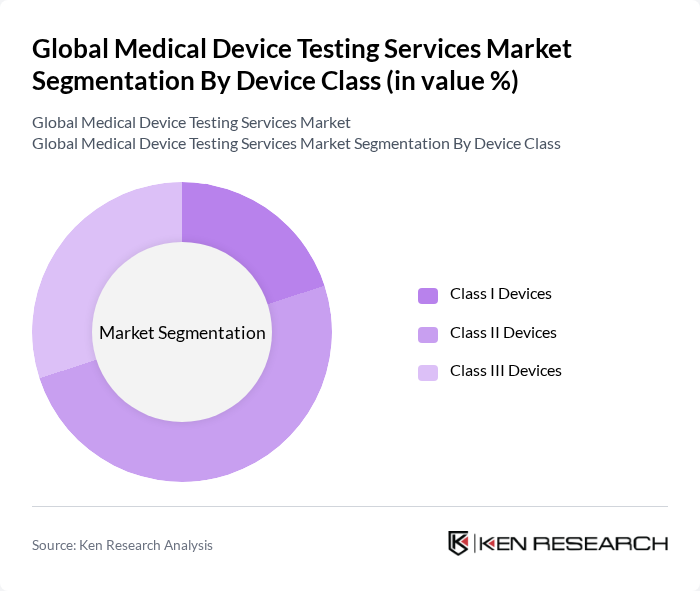
By Device Class:The device class segmentation categorizes medical devices into three classes based on the level of risk associated with their use. The subsegments include Class I Devices, Class II Devices, and Class III Devices. Class II Devices dominate the market due to their widespread use and the necessity for regulatory compliance, which drives demand for testing services to ensure safety and effectiveness. The growing complexity of Class II and III devices further accelerates demand for advanced testing protocols .
The Global Medical Device Testing Services Market is characterized by a dynamic mix of regional and international players. Leading participants such as SGS S.A., Eurofins Scientific SE, Intertek Group plc, Charles River Laboratories International, Inc., Laboratory Corporation of America Holdings (Labcorp), TÜV SÜD AG, Bureau Veritas S.A., NAMSA (North American Science Associates Inc.), WuXi AppTec Co., Ltd., Sotera Health Company (Sterigenics), Element Materials Technology, Pace Analytical Services LLC, BSI Group, UL LLC, American Preclinical Services contribute to innovation, geographic expansion, and service delivery in this space.
The future of the medical device testing services market appears promising, driven by ongoing technological advancements and increasing regulatory scrutiny. As manufacturers seek to enhance product safety and efficacy, the integration of AI and automation in testing processes will likely become more prevalent. Additionally, the shift towards remote testing solutions is expected to gain traction, allowing for greater flexibility and efficiency in testing practices, ultimately shaping the industry's landscape in the coming years.
| Segment | Sub-Segments |
|---|---|
| By Service Type | Preclinical Testing Services Clinical Testing Services Post-Market Surveillance Testing Regulatory Compliance Testing Biocompatibility Testing Sterilization Validation Package Testing Chemical Testing Electrical Safety Testing Mechanical Testing |
| By Device Class | Class I Devices Class II Devices Class III Devices |
| By Testing Phase | Preclinical Phase Clinical Phase Post-Market Phase |
| By End-User | Medical Device Manufacturers Contract Research Organizations (CROs) Regulatory Bodies Academic and Research Institutions Others |
| By Device Type | In-Vitro Diagnostic Devices Cardiovascular Devices Orthopedic Devices Imaging and Monitoring Devices Dental Devices Surgical Instruments Others |
| By Region | North America Europe Asia-Pacific Latin America Middle East & Africa |
| By Mode of Delivery | In-House Testing Outsourced Testing |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| In-vitro Diagnostic Testing Services | 65 | Laboratory Managers, Quality Control Specialists |
| Biocompatibility Testing Services | 50 | Regulatory Affairs Managers, R&D Scientists |
| Electrical Safety Testing Services | 45 | Compliance Officers, Product Safety Engineers |
| Clinical Evaluation Services | 60 | Clinical Research Coordinators, Medical Affairs Directors |
| Performance Testing Services | 55 | Product Development Managers, Testing Laboratory Directors |
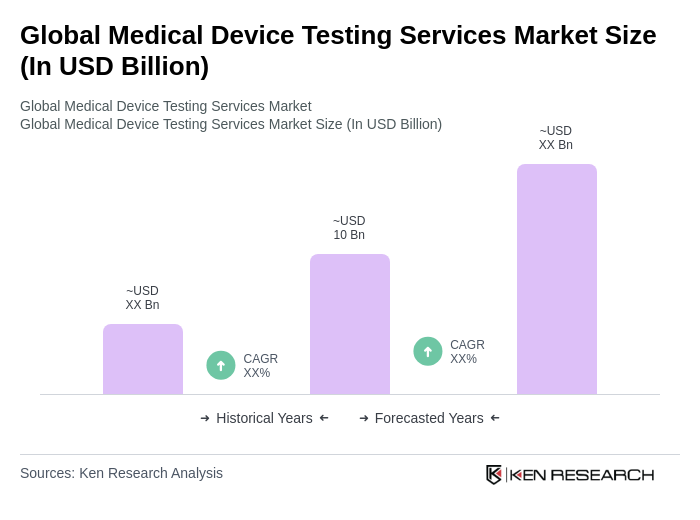
The Global Medical Device Testing Services Market is valued at approximately USD 10 billion, driven by the increasing demand for innovative medical devices, stringent regulatory requirements, and a focus on patient safety and product efficacy.