Region:Asia
Author(s):Geetanshi
Product Code:KRAC8255
Pages:87
Published On:November 2025
By Clinical Phase:
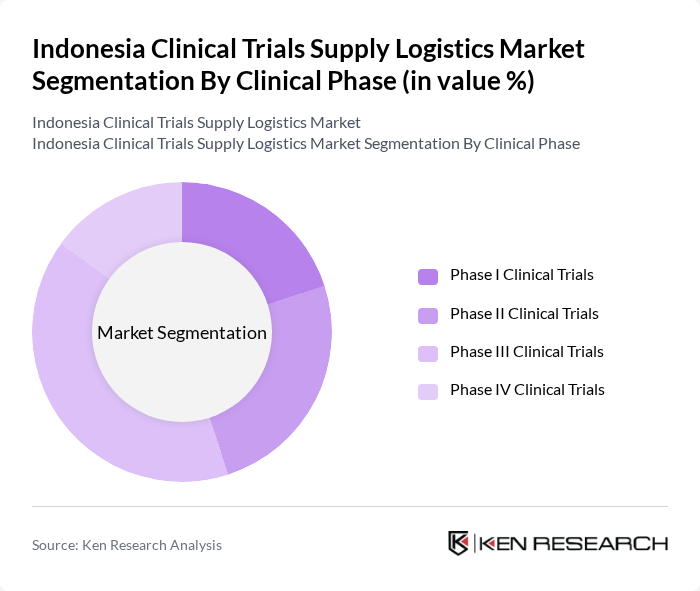
The clinical phase segmentation includes Phase I, Phase II, Phase III, and Phase IV clinical trials. Among these, Phase III Clinical Trials dominate the market due to their critical role in evaluating the efficacy and safety of new treatments before they receive regulatory approval. The increasing number of pharmaceutical companies conducting extensive Phase III trials to bring innovative therapies to market, along with the growing complexity and scale of these trials, is a significant driver of this segment's growth. The demand for specialized logistics solutions is particularly high in Phase III due to the need for large-scale distribution and stringent regulatory compliance .
By End-User:
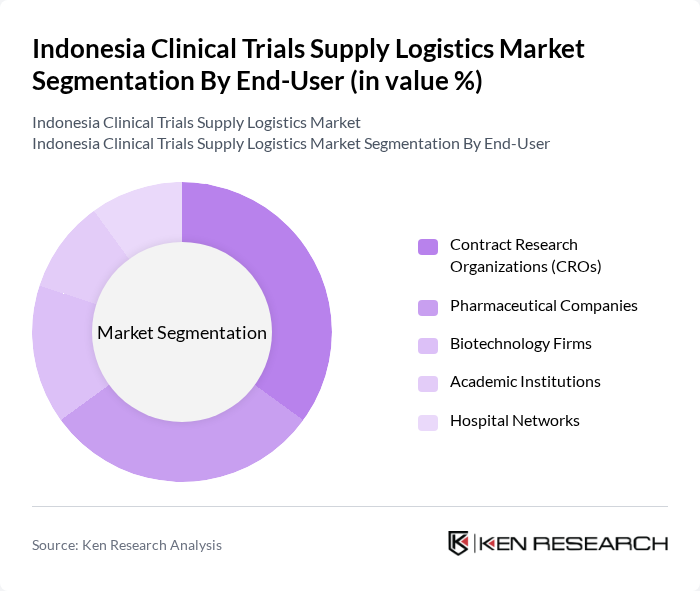
This segmentation includes Contract Research Organizations (CROs), Pharmaceutical Companies, Biotechnology Firms, Academic Institutions, and Hospital Networks. Contract Research Organizations (CROs) lead the market as they provide essential services for clinical trials, including logistics management, regulatory support, and supply chain optimization. The increasing reliance on CROs by pharmaceutical companies to streamline their clinical trial processes, reduce operational complexity, and ensure regulatory compliance is a key factor contributing to this segment's dominance. CROs are also at the forefront of adopting digital technologies and cold chain solutions to support complex, multi-site trials .
The Indonesia Clinical Trials Supply Logistics Market is characterized by a dynamic mix of regional and international players. Leading participants such as PT. Kimia Farma Logistics, PT. Indofarma Logistics Division, PT. Bio Farma Supply Chain, PT. Kalbe Farma Distribution, DHL Supply Chain Indonesia, FedEx Express Indonesia, PT. Sari Tani Logistics, PT. Medisafe Technologies, PT. Darya-Varia Laboratoria, PT. Sanbe Farma, PT. Phapros Logistics, PT. Soho Global Health Supply Chain, PT. Anugerah Pharmindo Lestari, PT. Mitra Keluarga Healthcare Logistics, PT. Dexa Medica Distribution contribute to innovation, geographic expansion, and service delivery in this space.
The future of Indonesia's clinical trials supply logistics market appears promising, driven by technological advancements and a growing emphasis on patient-centric approaches. As the industry shifts towards decentralized clinical trials, logistics providers will need to adapt to new operational models. Additionally, the integration of AI and data analytics is expected to enhance supply chain efficiency, while sustainability initiatives will play a crucial role in shaping logistics strategies, ensuring compliance with evolving regulations and market demands.
| Segment | Sub-Segments |
|---|---|
| By Clinical Phase | Phase I Clinical Trials Phase II Clinical Trials Phase III Clinical Trials Phase IV Clinical Trials |
| By End-User | Contract Research Organizations (CROs) Pharmaceutical Companies Biotechnology Firms Academic Institutions Hospital Networks |
| By Supply Chain Model | Centralized Supply Chain Decentralized Supply Chain Hybrid Supply Chain |
| By Temperature Control Requirements | Ambient Temperature (15-25°C) Refrigerated Temperature (2-8°C) Frozen Temperature (-20°C and below) Ultra-Cold Chain (-70°C and below) |
| By Geographic Coverage | Urban Areas Semi-Urban Areas Rural Areas Remote Locations |
| By Service Type | Cold Chain Logistics Inventory Management Distribution Management Packaging and Labeling Services Last-Mile Delivery |
| By Regulatory Compliance | Local Regulations (BPOM Compliance) International Standards (GDP, ICH-GCP) Quality Assurance and Traceability |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Pharmaceutical Logistics for Clinical Trials | 100 | Logistics Managers, Clinical Trial Coordinators |
| Supply Chain Management in CROs | 60 | Operations Managers, Supply Chain Analysts |
| Regulatory Compliance in Clinical Trials | 50 | Regulatory Affairs Specialists, Quality Assurance Managers |
| Patient Recruitment and Retention Strategies | 40 | Clinical Research Associates, Patient Engagement Officers |
| Logistics Challenges in Remote Areas | 50 | Field Operations Managers, Regional Logistics Coordinators |
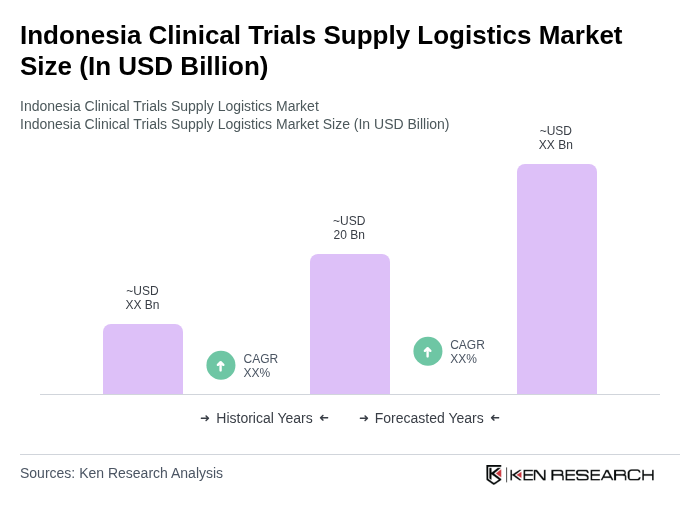
The Indonesia Clinical Trials Supply Logistics Market is valued at approximately USD 20 billion, driven by an increase in clinical trials, healthcare investments, and the demand for efficient supply chain solutions in the pharmaceutical sector.