Region:Asia
Author(s):Dev
Product Code:KRAD5312
Pages:97
Published On:December 2025
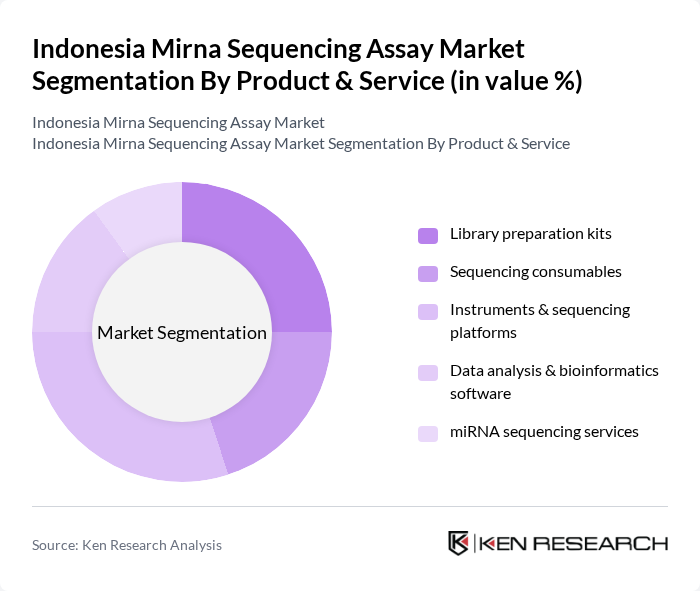
By Product & Service:The product and service segmentation of the Indonesia Mirna Sequencing Assay Market includes various components essential for conducting miRNA sequencing. The subsegments are Library preparation kits, Sequencing consumables, Instruments & sequencing platforms, Data analysis & bioinformatics software, and miRNA sequencing services. Each of these subsegments plays a crucial role in the overall market, with specific applications and demand drivers.
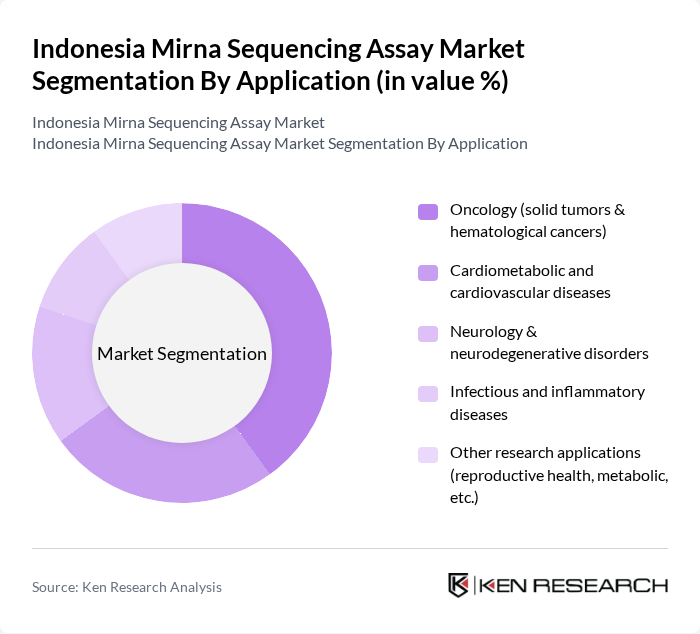
By Application:The application segmentation of the Indonesia Mirna Sequencing Assay Market encompasses various fields where miRNA sequencing is utilized. The subsegments include Oncology (solid tumors & hematological cancers), Cardiometabolic and cardiovascular diseases, Neurology & neurodegenerative disorders, Infectious and inflammatory diseases, and Other research applications (reproductive health, metabolic, etc.). Each application area has distinct requirements and growth potential.
The Indonesia Mirna Sequencing Assay Market is characterized by a dynamic mix of regional and international players. Leading participants such as Illumina, Inc., Thermo Fisher Scientific Inc., QIAGEN N.V., Agilent Technologies, Inc., F. Hoffmann-La Roche Ltd (Roche Diagnostics), PerkinElmer, Inc. (Revvity, Inc.), Takara Bio Inc., BGI Genomics Co., Ltd., Macrogen Inc., PT Kalbe Genexine Biologics, PT Prodia Widyahusada Tbk (Prodia Clinical Laboratory), PT Indogen Intervensi Genetik Indonesia (Indogen), Novogene Co., Ltd., 10x Genomics, Inc., Oxford Nanopore Technologies Ltd. contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Mirna sequencing assay market in Indonesia appears promising, driven by increasing investments in healthcare infrastructure and a growing emphasis on precision medicine. As the government implements national genomics policies, the regulatory environment is expected to improve, facilitating faster approval processes for new assays. Additionally, the integration of artificial intelligence in data analysis will enhance the efficiency of sequencing technologies, paving the way for innovative applications in diagnostics and treatment strategies.
| Segment | Sub-Segments |
|---|---|
| By Product & Service | Library preparation kits Sequencing consumables Instruments & sequencing platforms Data analysis & bioinformatics software miRNA sequencing services |
| By Application | Oncology (solid tumors & hematological cancers) Cardiometabolic and cardiovascular diseases Neurology & neurodegenerative disorders Infectious and inflammatory diseases Other research applications (reproductive health, metabolic, etc.) |
| By Technology | Sequencing by synthesis (NGS platforms) Ion semiconductor sequencing Nanopore sequencing Other technologies (SOLiD, SMRT, Sanger, microarray) |
| By Workflow | Library preparation Sequencing Data analysis & storage |
| By End-User | Research and academic institutes Hospital and clinical laboratories Pharmaceutical & biotechnology companies Independent sequencing service providers Others (government agencies, CROs) |
| By Region | Java (Jakarta, West Java, Central Java, East Java, Yogyakarta) Sumatra Kalimantan Sulawesi Bali & Nusa Tenggara Papua & Maluku |
| By Investment Source | Government funding (national & provincial) Private hospital and laboratory investments Pharmaceutical & biotech R&D budgets International grants and development agencies Venture capital & corporate investors |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Clinical Laboratories | 120 | Laboratory Directors, Geneticists |
| Healthcare Providers | 90 | Oncologists, Cardiologists |
| Research Institutions | 80 | Research Scientists, Biotechnologists |
| Diagnostic Companies | 70 | Product Managers, R&D Heads |
| Regulatory Bodies | 60 | Policy Makers, Compliance Officers |
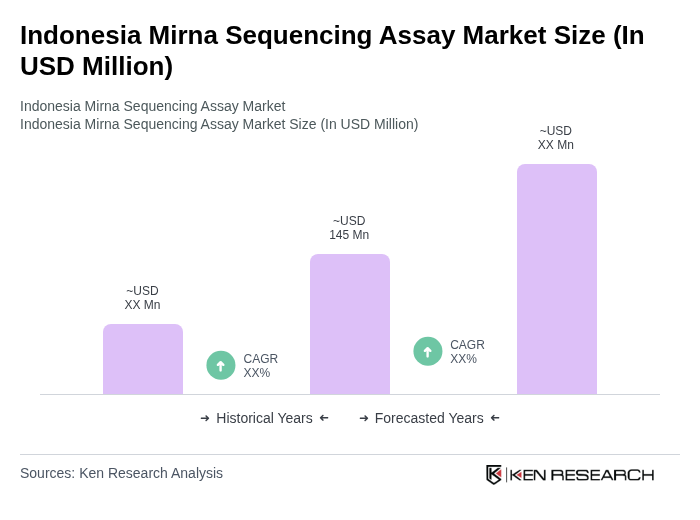
The Indonesia Mirna Sequencing Assay Market is valued at approximately USD 145 million, reflecting a significant growth driven by advancements in sequencing technologies and increasing healthcare expenditures focused on personalized medicine and biomarker discovery.